MnO2 nanoflakes grown on 3D graphite network for enhanced electrocapacitive performance†
Abstract
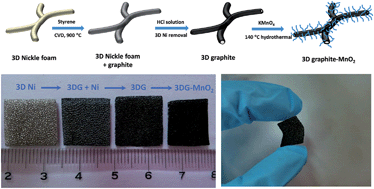
Free-standing 3D graphite (3DG) with an interconnected porous network and a highly conductive backbone was prepared by a chemical vapor deposition technique with Ni foam as the sacrificial template and styrene as the carbon precursor. The 3D graphite network serves as an excellent scaffold to grow MnO2 nanoflakes through a hydrothermal reaction between 3DG and KMnO4 aqueous solution. The interconnected and conductive 3D graphite network offers a pathway for fast electron and ion transport, while the MnO2 nanoflakes that emanate from the backbone surface of 3DG minimize the interfacial contact resistance between 3DG and MnO2, favoring the effective electron transport from the 3DG skeleton to MnO2 nanoflakes. As a result, the 3DG–MnO2 composite electrode with 13% MnO2 nanoflakes exhibited a specific capacitance of 210 F g−1 at a constant discharge current density of 2.0 A g−1, which still remained at 202 F g−1 at a high current density of 15 A g−1. Moreover, a good capacitance retention of 75% and an outstanding coulombic efficiency of 97.8% were achieved after 4000 cycles of galvanostatic charge–discharge. These superior capacitive properties make the 3DG–MnO2 composite one of the promising electrodes for electrochemical energy storage.

 Please wait while we load your content...
Please wait while we load your content...