Polyiminofluorene with conjugated benzimidazolylpyridine substituent groups: optical properties, ionochromism and coordinative self-assembly into electrochromic films†
Kalie Cheng and
Bernd Tieke*
Department of Chemistry, University of Cologne, Luxemburger Str. 116, D-50939 Cologne, Germany. E-mail: tieke@uni-koeln.de
First published on 27th May 2014
Abstract
Synthesis and characteristic properties of polyiminofluorene P1 with 2,6-bis(1′-methylbenzimidazolyl)pyridine (bip) ligands attached to the N-atoms of the backbone via conjugated 4-ethinylenephenylene spacer units are reported. P1 was obtained upon Pd-catalyzed amination of 2,7-dibromo-9,9-dihexylfluorene with 4-(2-(2,6-bis(1′-methylbenzimidazolyl)pyridinyl)-ethinyl)aniline. MALDI-TOF spectrometry shows that oligomers up to the tetradecamer are formed, the hexamer being the most abundant species. P1 is soluble in organic solvents such as xylene, toluene, or chlorobenzene forming yellow solutions with blue, green or yellow fluorescence depending on the polarity of the solvent. The fluorescence quantum yield in xylene is 41%. UV/Vis titration of the polymer with zinc chloride in toluene–methanol (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) indicates formation of 2
1) indicates formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand–metal (bis) complexes and 1
1 ligand–metal (bis) complexes and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mono complexes. Complex formation with various divalent transition metal ions is accompanied by ion specific colour changes (ionochromism) and quenching of the polymer fluorescence. The coordinative interactions between the bip ligands and metal ions such as Zn(II) or Cu(II) have been used for layer-by-layer assembly of ultrathin films on solid supports. Sequential adsorption of metal ions and P1 leads to formation of ultrathin coordination polymer network films. Due to ion specific metal-to-ligand charge transfer the P1/Zn(II) films are orange, while P1/Cu(II) films are purple. If anodically oxidized up to 750 mV vs. FOC, P1/Zn(II) and P1/Cu(II) films change colour into blue due to oxidation of the polymer backbone. Colour changes are reversible. Films prepared upon 12 dipping cycles exhibit switching times from the neutral to the oxidized state of 300 to 500 ms, the contrast being up to 53%. SEM studies indicate a homogeneous surface, which becomes slightly inhomogeneous after electrochemical cycling.
1 mono complexes. Complex formation with various divalent transition metal ions is accompanied by ion specific colour changes (ionochromism) and quenching of the polymer fluorescence. The coordinative interactions between the bip ligands and metal ions such as Zn(II) or Cu(II) have been used for layer-by-layer assembly of ultrathin films on solid supports. Sequential adsorption of metal ions and P1 leads to formation of ultrathin coordination polymer network films. Due to ion specific metal-to-ligand charge transfer the P1/Zn(II) films are orange, while P1/Cu(II) films are purple. If anodically oxidized up to 750 mV vs. FOC, P1/Zn(II) and P1/Cu(II) films change colour into blue due to oxidation of the polymer backbone. Colour changes are reversible. Films prepared upon 12 dipping cycles exhibit switching times from the neutral to the oxidized state of 300 to 500 ms, the contrast being up to 53%. SEM studies indicate a homogeneous surface, which becomes slightly inhomogeneous after electrochemical cycling.
1. Introduction
Coordinative self assembly of organic compounds and inorganic transition metal salts has been proven to be a suitable method for preparation of coordination polymers with interesting materials properties.1–8 Photo- and electroluminescent,9 electrochromic,10,11 and photovoltaic polymers can be prepared.12 If carried out on a solid substrate in a step-by-step approach, alternating self-assembly of organic and inorganic compounds can be used to build up films of coordination polymers with precisely controlled thickness in the nanometer range.13,14 Several examples of multiple sequential assembly of ditopic organic ligands and transition metal salts have been reported to result in functional films useful in electronics, optoelectronics, or as sensors.13,15–17Recent studies have demonstrated that the use of oligo- or polytopic ligands is a promising alternative to ditopic ligands.18,19 Ditopic ligands exhibit some disadvantages, e.g., they are easily desorbed from the substrate, sometimes tend to crystallize, and do not allow for healing of defects during the layer-by-layer deposition of ultrathin films. Alternating assembly of metal salts and polymers with ligand groups attached to the backbone, so-called polytopic ligands, is better suited, because stable films of coordination polymer networks well-adhering to the surface are obtained (Scheme 1). Using this approach electrochromic and photoluminescent films were recently described.20,21 It was shown that a structural variation of the polymer,22 the metal ions,23 and counterions24 can be used to tune-up and optimize the optical and electronic properties of the films.
 | ||
| Scheme 1 Preparation of monomer M1 and polymer P1. (i) Pd(PPh3)2Cl2, CuI, TEA, 3.5 h in DMF at 90 °C; (ii) Pd2(dba)3, Pt(Bu)3, NaOtBu, 16 h in toluene at 100 °C. | ||
Up to now mainly polymers substituted with terpyridine (tpy) ligands were used for preparation of electrochromic films. Only one example of a polymer with 2,6-bis(1′-methylbenzimidazolyl)pyridine (bip) ligands attached to the conjugated main chain via alkylene spacer units has been reported up to the present.21 While the preparation of tpy ligands is rather time-consuming and costly, bip ligands can be synthesized more easily on a large scale.25 Therefore it was of interest to prepare a new polytopic ligand consisting of a conjugated main chain and conjugated bip units attached to it. Since we were interested in electrochromic films we prepared P1 (Scheme 1) with easily oxydizable iminofluorene backbone and bip units attached to the N-atoms of the backbone via conjugated spacer units. P1 was synthesized upon palladium-catalyzed coupling of 2,7-dibromo-9,9-dihexylfluorene and 4-ethinylaniline comonomers.26–28,30
In our contribution, preparation and characteristic properties of P1 are reported, as well as studies of metal ion complexation, ionochromism, coordinative layer-by-layer (lbl) assembly into ultrathin films, and electrochromic properties of the films. Analytical studies were carried out using spectroscopic and microscopic methods such as NMR, UV and fluorescence spectroscopy, SEM, and cyclic voltammetry. It is demonstrated that P1 is an interesting new material suitable for metal ion complexation and sensing, and for preparation of ultrathin electrochromic films via coordinative self-assembly.
2. Experimental part
2.1 Materials
Copper chloride dihydrate (CuCl2·2H2O), iron(III) chloride hexahydrate (FeCl3·6H2O), iron(II) perchlorate hydrate(Fe(ClO4)2·xH2O) and zinc chloride (ZnCl2) were obtained from Acros. Manganese(II) chloride tetrahydrate (MnCl2·4H2O) and cobalt chloride hexahydrate (CoCl2·6H2O) were obtained from Merck. Nickel chloride hexahydrate (NiCl2·6H2O) was obtained from Fluka. Dithranole, silver tetrafluoroborate (AgBF4), phosphorus(V) oxybromide (OPBr3) and potassium hexafluorophosphate (KPF6) were obtained from Aldrich. All salts were used without further purification. The solvents were obtained from Aldrich with high purity grade and used without further purification. Milli-Q water was used for all experiments. 2,6-Bis-(1′-methylbenzimidazolyl)-4-hydroxypyridine (1) and 2,7-dibromo-9,9-dihexylfluorene (M2) were prepared according to the literature.31,322.2 Synthesis
1H-NMR (DMSO-d6, 300 MHz): δ (ppm) 4.31 (s, methyl, 6H); 7.53 (m, phenylene, 4H); 7.9 (dd, phenylene, 4H); 8.7 (s, pyridine, 2H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to yield 660 mg of the clean product. Yield: 86%, m.p.: >290 °C.
2) to yield 660 mg of the clean product. Yield: 86%, m.p.: >290 °C.1H-NMR (DMSO-d6, 300 MHz): δ (ppm) 4.29 (s, methyl, 6H); 5.86 (s, amine, 2H); 6.62 (d, aniline, 2H); 7.37 (m, phenylene, 4H); 7.40 (d, aniline, 2H); 7.75 (dd, phenylene, 4H); 8.35 (s, pyridine, 2H).
UV (DMSO): 353 nm; ε(353 nm) = 8636 l mol−1 cm−1. PL(toluene): 453 nm, excitation at 353 nm.
1H-NMR (CHCl3-d1, 300 MHz): δ (ppm) 0.4–2.0 (alkyl chain, 26H); 4.27 (s, methyl, 6H); 7.14 (d, aniline, 2H) 7.3 (m, phenylene, 4H); 7.37 (m, phenylene, 4H); 7.55 (d, phenyl-H, 2H); 7.75 (dd, phenyl-H, 4H); 7.91 (d, aniline, 2H), 8.53 (s, pyridine, 2H).
UV(toluene): 320 nm, 357 nm, 415 nm; ε(353 nm) = 15354 l mol−1 cm−1. PL(toluene): 496 nm, excitation at 357 nm.
2.3 Substrates
Quartz substrates (size 30 × 12 × 1 mm3) were cleaned upon immersion in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of 98% H2SO4 and 30% H2O2 7
3 mixture of 98% H2SO4 and 30% H2O2 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for 1 h (caution: the mixture is strongly oxidizing and may detonate upon contact with organic material). After multiple washing with Milli-Q water, the substrates were subjected to ultrasonication in alkaline isopropanol at 60 °C. Then the substrates were silanized with 3-aminopropyl(diethoxy)methylsilane (5%) in toluene, and finally precoated with three layer pairs of polystyrenesulfonate (PSS) and polyethyleneimine (PEI) as described in the literature.33 The precoating was completed with a layer of PSS. Indium tin oxide (ITO) coated glass substrates were cleaned by subjecting the substrates to ultrasonication in a mixture of water and ethanol (1
3 for 1 h (caution: the mixture is strongly oxidizing and may detonate upon contact with organic material). After multiple washing with Milli-Q water, the substrates were subjected to ultrasonication in alkaline isopropanol at 60 °C. Then the substrates were silanized with 3-aminopropyl(diethoxy)methylsilane (5%) in toluene, and finally precoated with three layer pairs of polystyrenesulfonate (PSS) and polyethyleneimine (PEI) as described in the literature.33 The precoating was completed with a layer of PSS. Indium tin oxide (ITO) coated glass substrates were cleaned by subjecting the substrates to ultrasonication in a mixture of water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 30 min each. Subsequently the substrates were precoated with three layer pairs of (PSS) and (PEI).
1) for 30 min each. Subsequently the substrates were precoated with three layer pairs of (PSS) and (PEI).
2.4 Film preparation
The multilayers were deposited on quartz and ITO substrates using multiple sequential coordinative layer-by-layer assembly. The precoated substrates were alternately dipped into solutions of the transition metal salts and P1. Solutions of 5 × 10−3 M metal hexafluorophosphates in toluene–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and a solution of 8.5 × 10−5 M P1 in toluene–methanol (2
3) and a solution of 8.5 × 10−5 M P1 in toluene–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were used for the film preparation. The metal hexafluorophosphate solutions were prepared by adding 2 eq. of potassium hexafluorophosphate in 1 ml DMF to the 5 × 10−3 M solution of the corresponding metal chloride in toluene–methanol (2
3) were used for the film preparation. The metal hexafluorophosphate solutions were prepared by adding 2 eq. of potassium hexafluorophosphate in 1 ml DMF to the 5 × 10−3 M solution of the corresponding metal chloride in toluene–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After each dipping step the substrates were cleaned upon immersion in the pure toluene–methanol (2
3). After each dipping step the substrates were cleaned upon immersion in the pure toluene–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent mixture.
3) solvent mixture.
2.5 Instrumentation
UV-Vis-spectra were measured on a Perkin-Elmer Lambda 14 spectrometer. The baseline was corrected by subtracting the spectrum of a quartz substrate precoated with 3 layer pairs of PEI and PSS. Photoluminescence spectra were recorded on a Perkin-Elmer LS 50 B spectrometer. Photoluminescence quantum yields were measured in xylene, toluene and chlorobenzene solutions. The values were calculated by comparing with rhodamin 6G in ethanol (εR = 0.95). MALDI-TOF experiments were carried out on a Bruker Daltonics Autoflex spectrometer. A dithranole matrix was used to which silver tetrafluoroborate was added. Cyclovoltammetric studies were carried out on a HEKA PG 390 potentiostat using Potpulse-software for data acquisition and potentiostat control. The experiments were carried out in a conventional three-electrode electrochemical cell at room temperature. Polymer coated ITO-substrates were used as working electrodes, platinum was used as counter and reference electrodes. A 0.1 M tetrabutylammonium hexafluorophosphate solution in acetonitrile saturated with nitrogen was used as electrolyte medium. SEM images were obtained using a Zeiss Neon 40 scanning electron microscope. EDX measurements were carried out on an INCA Drycool device connected to a Zeiss Neon 40 SEM. The images were obtained with an acceleration voltage of 20 kV over an area of 2500 μm2. The film thickness was measured using a Vecco DekTak3. Multilayer films on quartz substrates were partially scratched from the substrate. The height profile was scanned with an error of ±2.5 nm.3. Results and discussion
3.1 Preparation of P1
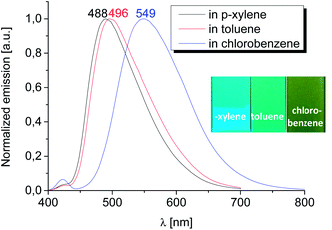
P1 was synthesized upon palladium-catalyzed Buchwald–Hartwig polycondensation of 2,7-dibromo-9,9-dihexylfluorene (M2) and M1. M1 was obtained via Sonogashira-coupling of 4-ethinylaniline (3) and 2. 2 was synthesized upon bromination of 1, whereas 1 was obtained via a well known condensation reaction of chelidamic acid with N-methyl-phenylene-2,3-diamine.31 Pd2(dba)3/tri-tert-butylphosphine was used as catalyst for polymerization, and sodium t-butoxide was used as base. The product was obtained as a yellow powder, which was soluble in common organic solvents like toluene, THF or DCM. The molecular weight distribution of P1 was determined using MALDI-TOF spectrometry. The spectrum is shown in the ESI (Fig. S1†). A distribution of the absolute molecular weights of the different chain lengths was obtained. The assigned molecular weights correspond to exact multiples of the repeat unit of 785 m/z. The intense peak of 6 repeat units represents the most abundant chain length indicating that P1 is actually an oligomer. A maximum of 14 repeat units could be detected. Fig. 1 shows the UV-Vis absorption spectrum of P1 in toluene.P1 exhibits absorption maxima at 319 and 358 nm originating from the conjugated main chain, and another one at 417 nm originating from the conjugated ligand in the side chain. The fluorescence spectra in different solvents are shown in Fig. 2.
The emission is shifted from blue through greenish to yellow with maxima at 488, 496 and 549 nm, if either xylene, toluene or chlorobenzene are used as solvent. The solvatochromism originates from polymer–solvent interactions, in which solvent polarity, dipolarity, and the ability of the solvent to act as hydrogen bond donor or acceptor play a major role. The higher the dipolarity of the solvent, the more the absorption and fluorescence maxima are bathochromically shifted. The maxima differ by 61 nm depending on whether the non-polar p-xylene or the highly polar chlorobenzene are used as solvents. With increasing polarity of the solvent, the fluorescence quantum yield becomes lower. In p-xylene the quantum yield is 41%, in toluene and chlorobenzene 39 and 19%, respectively.
3.2 Metal ion complexation of P1 with various metal salts
If a transition metal salt is added to the solution of P1, a metal ion complex is formed as indicated by a colour change of the solution from yellow to brownish red or orange. Simultaneously the fluorescence of P1 is quenched. For a quantitative analysis of the metal ion complexation a solution of P1 was titrated with a divalent transition metal salt. For this purpose a solution of 1.43 × 10−6 monomolar P1 (monomoles = moles of repeat units) in toluene–methanol (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and 1.19 × 10−3 molar transition metal salt solutions in the same solvent mixture were used. After each addition of a few drops of the metal salt solution an absorption spectrum was measured. In Fig. 3, the colour of P1 and its various metal ion complexes in solution are shown. UV-Vis-spectra monitored during titration with NiCl2, CoCl2, Fe(ClO4)2, FeCl3, and MnCl2 are shown in the ESI (Fig. S2–S9†). Colour and absorbance spectra of Fe(II) and Fe(III) complexes differ due to the formation of low- and high-spin complexes, respectively.29
1), and 1.19 × 10−3 molar transition metal salt solutions in the same solvent mixture were used. After each addition of a few drops of the metal salt solution an absorption spectrum was measured. In Fig. 3, the colour of P1 and its various metal ion complexes in solution are shown. UV-Vis-spectra monitored during titration with NiCl2, CoCl2, Fe(ClO4)2, FeCl3, and MnCl2 are shown in the ESI (Fig. S2–S9†). Colour and absorbance spectra of Fe(II) and Fe(III) complexes differ due to the formation of low- and high-spin complexes, respectively.29
 | ||
| Fig. 3 Photographic images of 1.43 × 10−6 monomolar solutions of P1 before and after complexation with 1 eq. of various metal salts. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a solution of zinc perchlorate in the same solvent mixture. Upon titration the low energy π–π* transition of the fluorene unit at 361 nm, and the bip absorption at 414 nm decreased. Simultaneously new maxima at 390 and 506 nm appeared, which can be ascribed to a metal-to-ligand charge transfer (MLCT) band, indicating the complexation of the metal ions with the bip-ligands (Fig. 4(a)). The complexation results in a colour change from yellow to orange.
1) with a solution of zinc perchlorate in the same solvent mixture. Upon titration the low energy π–π* transition of the fluorene unit at 361 nm, and the bip absorption at 414 nm decreased. Simultaneously new maxima at 390 and 506 nm appeared, which can be ascribed to a metal-to-ligand charge transfer (MLCT) band, indicating the complexation of the metal ions with the bip-ligands (Fig. 4(a)). The complexation results in a colour change from yellow to orange.
With each titration step the absorbance at 361 nm decreases until 0.5 equivalents of Zn(ClO4)2 are added (see inset of Fig. 4(a)). This indicates the formation of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (bis-complex) (see also Scheme 2). During the addition of the first 0.5 equivalents five isosbestic points at about 320, 346, 367, 406 and 460 nm occur, indicating an equilibrium between the bis-complex and the non-complexed ligands and ions. Further addition leads to formation of the mono-complexes as shown in Scheme 2. As shown in the inset of Fig. 4(b), the formation of the mono-complex is accompanied with a re-increase of the absorbance at 361 nm until a ligand-to-metal ratio of 1
1 complex (bis-complex) (see also Scheme 2). During the addition of the first 0.5 equivalents five isosbestic points at about 320, 346, 367, 406 and 460 nm occur, indicating an equilibrium between the bis-complex and the non-complexed ligands and ions. Further addition leads to formation of the mono-complexes as shown in Scheme 2. As shown in the inset of Fig. 4(b), the formation of the mono-complex is accompanied with a re-increase of the absorbance at 361 nm until a ligand-to-metal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is reached and the mono-complex is formed. The transition from the bis- into the mono-complex is accompanied with isosbestic points at about 320, 350 and 490 nm indicating an equilibrium between the two complexes. Formation of the bis-complex and the corresponding mono-complex could only be seen for titration of P1 with zinc perchlorate in polar solvents such as acetonitrile at 361 nm, whereas the titration with various metal chlorides, or metal perchlorate salts in non-polar solvents only led to an increase of the absorbance of the MLCT-band until the mono-complex was formed (see also ESI†).
1 is reached and the mono-complex is formed. The transition from the bis- into the mono-complex is accompanied with isosbestic points at about 320, 350 and 490 nm indicating an equilibrium between the two complexes. Formation of the bis-complex and the corresponding mono-complex could only be seen for titration of P1 with zinc perchlorate in polar solvents such as acetonitrile at 361 nm, whereas the titration with various metal chlorides, or metal perchlorate salts in non-polar solvents only led to an increase of the absorbance of the MLCT-band until the mono-complex was formed (see also ESI†).
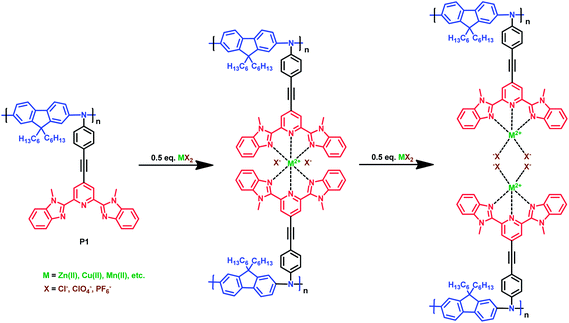 | ||
| Scheme 2 Complexation of P1 (left) with transition metal ions and formation of the bis-complex (middle) and mono-complex (right). | ||
The formation of the bis-complex very likely proceeds as an interchain reaction and should be accompanied by cross-linking, but a precipitation was not observed. This is probably due to the low molecular weight and the low concentration of P1 in the solution, which prevent an efficient cross-linking. Therefore the addition of metal ions only leads to formation of colloidal aggregates of the complexes. If polymer solutions with concentrations higher 0.05 mol l−1 were used, titration led to precipitation of the polymer–metal ion complex.
We also titrated P1 with zinc acetate in a mixture of toluene–methanol (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Again a colour change was observed due to the formation of the MLCT band. However, in this case far more of the metal salt had to be added than in case of zinc perchlorate or chloride until addition did not change the absorbance anymore (ESI†). The reason is that the dissociation of the metal ion complex is strongly dependent on the nature of the anion and the solvent used for the titration experiment.
1). Again a colour change was observed due to the formation of the MLCT band. However, in this case far more of the metal salt had to be added than in case of zinc perchlorate or chloride until addition did not change the absorbance anymore (ESI†). The reason is that the dissociation of the metal ion complex is strongly dependent on the nature of the anion and the solvent used for the titration experiment.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) a similar shift of the absorption bands was observed as for the titration with ZnCl2. New bands occurring at 391 and 517 nm are due to formation of the MLCT complex, whereas absorption bands of the polymer at 355 and 420 nm decrease in intensity. Three isosbestic points were formed at 367, 402 and 465 nm. The complexation is accompanied by a colour change from yellow to purple. The absorption at 517 nm increased linearly until 1.0 equivalent of CuCl2 was added. Additional metal ions had no further influence on the absorption spectra. UV-Vis-spectra monitored during titration with CuCl2 are shown in the ESI.†
1) a similar shift of the absorption bands was observed as for the titration with ZnCl2. New bands occurring at 391 and 517 nm are due to formation of the MLCT complex, whereas absorption bands of the polymer at 355 and 420 nm decrease in intensity. Three isosbestic points were formed at 367, 402 and 465 nm. The complexation is accompanied by a colour change from yellow to purple. The absorption at 517 nm increased linearly until 1.0 equivalent of CuCl2 was added. Additional metal ions had no further influence on the absorption spectra. UV-Vis-spectra monitored during titration with CuCl2 are shown in the ESI.†Fluorescence spectroscopy of the titration of P1 with a solution of CuCl2 in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile–chloroform solvent mixture revealed quenching of the fluorescence of P1 upon complexation with the metal ions (Fig. 5). The emission maximum at 542 nm decreases upon addition of the metal salt until at a metal-to-ligand ratio of 0.58 the emission is fully quenched. The quenching can be ascribed to the formation of the bis-complex in which an energy transfer from the conjugated system to the metal center leads to a static quenching of the fluorescence.
1 acetonitrile–chloroform solvent mixture revealed quenching of the fluorescence of P1 upon complexation with the metal ions (Fig. 5). The emission maximum at 542 nm decreases upon addition of the metal salt until at a metal-to-ligand ratio of 0.58 the emission is fully quenched. The quenching can be ascribed to the formation of the bis-complex in which an energy transfer from the conjugated system to the metal center leads to a static quenching of the fluorescence.
3.3 Coordinative layer-by-layer assembly and optical film properties
Using coordinative layer-by-layer-assembly described in Scheme 3 films of P1 with zinc and copper ions were deposited on pretreated quartz and ITO glass substrates.PF6− was added to the metal salt solutions in order to decrease the solubility of the complexes, and to improve the deposition of the films. The film formation was characterized by UV-Vis-spectroscopy. Absorption spectra were recorded at intervals of two dipping cycles.
With each dipping cycle, the absorbance of the films increased. Band shape and absorption maxima of the films are in agreement with those of the zinc and copper complexes in solution. In Fig. 6(a), absorption spectra of films of the P1–zinc hexafluorophosphate complex are shown. It can be seen that the absorbance of the π → π* transition at 390 nm increases linearly with the number of deposited layers. This indicates that in each dipping cycle the same amount of polymer and metal ions are deposited. After 12 dipping cycles the film exhibits an intense orange colour and appears very homogeneous. LbL-assembly of the polymer and copper hexafluorophosphate on a quartz substrate also led to film formation (Fig. 6(b)). A purple film with homogeneous colouration was obtained. Again the absorption increases linearly with the number of dipping cycles applied. Profilometric studies indicate that P1/Zn(II) films prepared upon 12 dipping cycles exhibit a thickness of 141 nm, whereas films of the P1–copper complex are 81 nm in thickness.
3.4 Electrochemical properties
In order to study the electrochemical properties, the coordination polymer films were built up on indium tin oxide(ITO)-coated glass substrates. Films of P1 and Zn(II) or Cu(II) ions were prepared. Their cyclic voltammograms are shown in Fig. 7. Two reversible anodic waves occur, which can be related to oxidation of the nitrogen atoms of the polymer main chains (Scheme 4). All films show highly reversible oxidation steps between 0.6 and 0.9 V. The expected second wave does not always appear due to overlap with the first wave. The same is observed for the subsequent reduction. Films of P1/Zn(II) exhibit anodic waves at 0.40 and 0.66 V. The subsequent reductive wave appears at 0.12 V. In films of P1/Cu(II) the two reversible anodic waves are observed at 0.44 and 0.60 V. The related reductive waves occur at 0.41 and 0.29 V. | ||
| Scheme 4 Change in the conjugated π-system of P1 upon first and second oxidation. Cation radicals, dications and quinoid structures are formed. | ||
3.5 Electrochromic behaviour
Anodic oxidation and subsequent reduction of the coordination polymer films are accompanied with reversible colour changes. The change in the absorption behaviour originates from oxidation of the polymer as indicated in Scheme 4. A spectro-electrochemical study of the colour changes is shown in Fig. 8.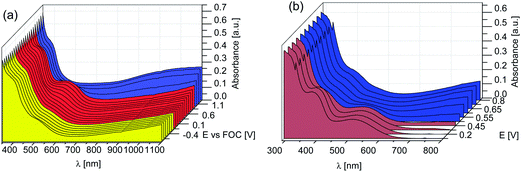 | ||
| Fig. 8 Spectro-electrochemical measurements of films of P1/Zn(II) (a) and P1/Cu(II) (b), (12 dipping cycles). | ||
A conventional electrochemical three-electrode cell was used, which was placed in a UV/Vis-spectrometer. The coordination polymer film deposited on an ITO-coated glass substrate was used as working electrode. Different potentials were applied and the absorption spectra were measured. At low potential films of P1/Zn(II) exhibit an orange colour, which turns into red at 0.8 V, and from red to blue at 1.1 V. The first colour change originates from an increase of the absorbance at 503 nm due to formation of cation radicals in the backbone. It involves an isosbestic point at 470 nm. The second colour change goes along with a decrease of the absorbance at 503 nm and a broad increase of the absorbance from the isosbestic point at 576 nm to the infrared. It can be ascribed to formation of the dication state with a quinonoid structure in the backbone as shown in Scheme 4. Photographic images of the neutral and the fully oxidized film are shown in Fig. 9(a).
 | ||
| Fig. 9 Photographic images of the neutral form and the second oxidation state of films of P1 and Zn(II) (a) and Cu(II) (b), (24 dipping cycles). | ||
Spectroelectrochemical measurements of films of P1/Cu(II) are shown in Fig. 8(b). As the film in its neutral form already exhibits a reddish colour, the first oxidation has no visible effect on the absorption behaviour. The second oxidation at 0.65 V involves a colour change from purple to blue (Fig. 9(b)), which again can be ascribed to the formation of the dipolaron state exhibiting a broad absorption ranging from 580 nm to the infrared. Simultaneously the absorption bands of the neutral chromophore at 380 and 515 nm are decreased. At a voltage above 0.5 V the isosbestic point is shifted from 612 to 580 nm.
For the P1/Zn(II) and P1/Cu(II) films we also studied switching time, contrast and long term stability. For the determination of the long term stability, the transmission at 800 nm was recorded vs. time while the films were switched between 0.0 and 0.9 V (a) and 0.0 and 0.8 V (b) every 10 seconds. The first 40 switching cycles are shown in Fig. 10(a) and (b). Switching times were determined by a linear fit of the change of absorbance as shown in Fig. 10(c) and (d). The contrast was determined from the change in transmission, Δ%T, at 800 nm obtained upon switching from the neutral to the oxidized state. The experiments were carried out with 12 double layer films (Fig. 10). The data are listed in Table 1. It turned out that the freshly prepared films exhibit switching times of 300 to 500 ms. The contrast originally is 53%, and is reduced to about 30% after 40 switching cycles. This may originate from partial desorption of the film from the substrate. The total reduction of the contrast was lowest for films with Cu(II) due to a stronger metal–ligand interaction than in the Zn-based film.34,35
| Film thickness [nm] | Contrast [%] at 800 nm | Switching time [ms] | |
|---|---|---|---|
| P1/Zn(II) film before electrochromic switching | 149 | 53 | 530 |
| P1/Zn(II) film after 40 switching cycles | 35 | 2600 | |
| P1/Cu(II) film before electrochromic switching | 81 | 36 | 320 |
| P1/Cu(II) film after 40 switching cycles | 29 | 800 |
3.6 Film composition and morphology
The film morphology was studied using REM and EDX prior and subsequent to electrochromic switching. EDX measurements indicate a decrease of the carbon-to-Zn(II) ratio after several switching cycles (Fig. 11). This indicates that during the switching process the polymer is partly removed from the substrate. Removal of parts of the films could also explain the loss in contrast. REM measurements indicate that the surface of the freshly prepared films is rather homogeneous with some humps distributed over the whole specimen (Fig. 12). After electrochemical switching the humps disappeared, but the surface generally became more inhomogeneous probably due to loss of material during the cycling process. It is especially evident at higher magnification. | ||
| Fig. 11 EDX measurements of films of P1/Zn(II) before (a) and after (b) electrochromic switching (up) and their quantitative results (down). | ||
4. Conclusions
First, our study indicates that a polytopic ligand P1 with iminofluorene backbone and 2,6-bis(1′-methyl-benzimidazolyl)-pyridine (bip) ligand groups attached to the backbone via conjugated spacer units can be synthesized upon a palladium catalyzed amination reaction. P1 is readily soluble in organic solvents.Second, P1 is able to form complexes with divalent transition metal salts such as zinc(II) and copper(II) ions in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Metal ion complexation proceeds under noticeable colour changes from yellow to orange or purple causing a strong ionochomism of P1. Presence of divalent transition metal salts quenches the photoluminescence. It is a strong effect, which can be used for sensing of transition metal ions in the ppm range.
1 stoichiometry. Metal ion complexation proceeds under noticeable colour changes from yellow to orange or purple causing a strong ionochomism of P1. Presence of divalent transition metal salts quenches the photoluminescence. It is a strong effect, which can be used for sensing of transition metal ions in the ppm range.
Third, our study shows that a proper choice of solvent and nature of the counterions enables a layer-by-layer assembly of polymer–metal salt complexes on solid substrates. Ultrathin coordination polymer network films are formed upon alternating assembly of divalent transition metal salts and P1, similar to the assembly of transition metal salts and polyiminofluorene,23 polycarbazolylene,22 and polyaniline derivatives36 with terpyridine ligands in the side chains reported recently.22,23
Fourth, the films are electrochromic, i.e., they exhibit reversible oxidation behaviour as well as reversible electrochromism, if the potential is switched between the neutral state at 0.0 V and the oxidized state at 0.8 V (Zn(II)) or 0.65 V (Cu(II)) vs. FOC/FOC+, respectively. The colour change depends on the kind of metal ion incorporated in the films. The electrochromical properties are similar to the tpy-based coordination polymer films reported earlier,22,23 but the different ligands and spacer units influence the metal-to-ligand charge transfer, and the strength of the ligand-to-metal ion binding leading to films of different colour and stability. On one hand, relatively thick films with high contrast and short switching times can be prepared, which is favourable for application in electrochromic devices, but on the other hand, long term stability is lower than for the tpy based systems22,23 and still has to be improved. REM and EDX measurements indicate that electrochromic switching is accompanied by partial desorption. This might be due to the low molecular weight of the polymer but a weaker complex strength may also play a role. Higher molecular weights and the use of an electrolyte gel instead of a solution might help to increase the stability and maintain contrast and switching times of the films.
References
- A. Wild, A. Winter, F. Schlütter and U. S. Schubert, Chem. Soc. Rev., 2011, 40, 1459 RSC.
- A. S. Abd-El-Aziz, C. E. Carraher and P. D. Horvey, in Macromolecules Containing Metal and Metal-Like Elements, Vol. 10: Photophysics and Photo-chemistry of Metal-Containing Polymers, ed. M. Zeldin, John Wiley & Sons, Hoboken, N. J, 2010 Search PubMed.
- P. Nguyen, P. Gómes-Elipe and I. Manners, Chem. Rev., 1999, 99, 1515 CrossRef CAS PubMed.
- (a) M. Rehahn, Acta Polym., 1998, 49, 201 CrossRef CAS; (b) U. Velten and M. Rehahn, Macromol. Chem. Phys., 1998, 199, 127 CAS.
- T. Kaliyappan and P. Kannan, Prog. Polym. Sci., 2000, 25, 343 CrossRef CAS.
- E. C. Constable, C. E. Housecroft, J. N. Lambert and D. A. Malarek, Chem. Commun., 2005, 3739 RSC.
- X. Wang and R. McHale, Macromol. Rapid Commun., 2010, 31, 331 CrossRef CAS PubMed.
- K. A. Williams, A. J. Boydston and C. W. Bielawski, Chem. Soc. Rev., 2007, 36, 729 RSC.
- Y.-Y. Chen, Y.-T. Tao and H.-C. Liu, Macromolecules, 2006, 39, 8559 CrossRef CAS.
- D. G. Kurth, J. P. Lopez and W.-F. Dong, Chem. Commun., 2005, 2119 RSC.
- F. S. Han, M. Higuchi and D. G. Kurth, J. Am. Chem. Soc., 2008, 130, 2073 CrossRef CAS PubMed.
- O. Hagemann, M. Jorgensen and F. C. Krebs, J. Org. Chem., 2006, 71, 5546 CrossRef CAS PubMed.
- B. Tieke, Curr. Opin. Colloid Interface Sci., 2011, 16, 499 CrossRef CAS PubMed.
- D. M. Sarno, B. Jiang, D. Grosfeld, J. O. Afriyie, W. Mationzo and W. E. Jones Jr, Langmuir, 2000, 16, 6191 CrossRef CAS.
- H. Krass, G. Papastravou and D. G. Kurth, Chem. Mater., 2003, 15, 196 CrossRef CAS.
- W. Zhao, B. Tong, Y. Pan, J. Shen, J. Zhi and J. Shi, et al., Langmuir, 2009, 25, 11796 CrossRef CAS PubMed.
- N. Tuccitto, I. Delfanti, V. Torrisi, F. Scandola, C. Chiorboli and V. Stepanenko, et al., Phys. Chem. Chem. Phys., 2009, 11, 4033 RSC.
- B. Belghoul, I. Welterlich, A. Maier, A. Toutianoush, A. R. Rabindranath and B. Tieke, Langmuir, 2007, 23, 5062 CrossRef CAS PubMed.
- M. Wanunu, A. Vaskevich, S. R. Cohen, H. Cohen, R. Arad-Yellin and A. Shanzer, et al., J. Am. Chem. Soc., 2005, 127, 17877 CrossRef CAS PubMed.
- A. Maier, A. R. Rabindranath and B. Tieke, Adv. Mater., 2009, 21, 959 CrossRef CAS.
- I. Welterlich and B. Tieke, Macromolecules, 2011, 44, 4194 CrossRef CAS.
- A. Maier, H. Fakhrnabavi, A. R. Rabindranath and B. Tieke, J. Mater. Chem., 2011, 21, 5795 RSC.
- A. Maier, A. R. Rabindranath and B. Tieke, Chem. Mater., 2009, 21, 3668 CrossRef CAS.
- A. Maier, K. Cheng, J. Savich and B. Tieke, ACS Appl. Mater. Interfaces, 2011, 3, 2710 CAS.
- J. B. Beck, J. M. Ineman and S. J. Rowan, Macromolecules, 2005, 38, 5060 CrossRef CAS.
- J.-F. Marcoux, S. Wagaw and S. L. Buchwald, J. Org. Chem., 1997, 62, 1568 CrossRef CAS.
- F. E. Goodson and J. F. Hartwig, Macromolecules, 1998, 31, 1700 CrossRef CAS.
- T. Kanbara, M. Oshima, T. Imayasu and K. Hasegawa, Macromolecules, 1998, 31, 8725 CrossRef CAS.
- A. W. Addison, S. Burman, C. G. Wahlgren, O. A. Rajan, T. M. Rowe and E. Sinn, J. Chem. Soc., 1987, 2621 CAS.
- A. R. Rabindranath, A. Maier, M. Schäfer and B. Tieke, Macromol. Chem. Phys., 2009, 210, 659 CrossRef CAS.
- S. J. Rowan and J. B. Beck, Faraday Discuss., 2005, 10, 3301 Search PubMed.
- J. K. Lee, G. Klaerner and R. D. Miller, Chem. Mater., 1997, 11, 11083 Search PubMed.
- M. Pyrasch, D. Amirbeyki and B. Tieke, Adv. Mater., 2001, 12, 1188 CrossRef.
- K. Kanaizuka and H. Nishihara, in Bottom-up Nanofabrication: Supramolecules, Self-Assemblies and Organized Films, Vol. 5 Organized Films, ed. K. Ariga and H. S. Nalwa, American Scientific Publishers, Stevenson Ranch, 2009, p. 429 Search PubMed.
- R. H. Holyer, C. D. Hubbard, S. F. A. Kettle and R. G. Wilkins, Inorg. Chem., 1966, 5, 622 CrossRef CAS.
- A. Maier and B. Tieke, J. Phys. Chem. B, 2012, 116, 925 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03969f |
| This journal is © The Royal Society of Chemistry 2014 |