One-pot synthesis of hierarchical mesoporous SnO2 spheres using a graft copolymer: enhanced photovoltaic and photocatalytic performance†
Jung Tae Parkab,
Chang Soo Leea and
Jong Hak Kim*a
aDepartment of Chemical and Biomolecular Engineering, Yonsei University, 262 Seongsanno, Seodaemun-gu, Seoul 120-749, South Korea. E-mail: jonghak@yonsei.ac.kr; Fax: +82-2-312-6401; Tel: +82-2-2123-5757
bDepartment of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., 66-325, Cambridge, MA 02139, USA
First published on 2nd July 2014
Abstract
We synthesized hierarchical mesoporous SnO2 (HM-SnO2) spheres with a large surface area (85.3 m2 g−1) via a one-pot controlled solvothermal process using tin chloride pentahydrate and graft copolymer, i.e., poly(vinyl chloride)-g-poly(oxyethylene methacrylate) (PVC-g-POEM) as a Sn precursor and structure directing agent, respectively. Solid-state dye-sensitized solar cells (ssDSSCs) fabricated with HM-SnO2 spheres on an organized mesoporous SnO2 interfacial (om-SnO2 IF) layer as the photoanode had a long-term stable efficiency of 3.4% at 100 mW cm2, which was much higher than that of ssDSSCs with a photoanode comprising nonporous SnO2 (NP-SnO2) spheres (1.9%). We attributed the enhanced device performance of ssDSSCs fabricated with the HM-SnO2 photoanode to the well-organized hierarchical structure with dual pores (23.5 and 162.3 nm), which provided a larger surface area, improved light scattering, and decreased charge recombination compared to the nonporous SnO2 (NP-SnO2) photoanode. We confirmed this by reflectance, incident photon to current conversion efficiency (IPCE), and intensity modulated photocurrent/voltage spectroscopy (IMPS/IMVS) measurements. Introduction of an om-SnO2 IF layer between the HM-SnO2 spheres and fluorine-doped tin oxide (FTO) substrate enhanced light harvesting, increased electron transport, reduced charge recombination, and decreased interfacial/internal resistance. Photocatalytic tests indicated that HM-SnO2 spheres showed high activity with good recyclability for photodegradation of methyl orange under UV light irradiation.
Introduction
Dye-sensitized solar cells (DSSCs) have attracted considerable research interest since the seminal study in 1991 by Gratzel and co-workers,1 because DSSCs have high efficiency, are low cost, easy to fabricate, have low manufacturing toxicity, are amenable to scale-up, and cell design is flexible. Several approaches have been developed to synthesize the individual components of DSSCs such as the photoanode,2–4 sensitizer,5,6 counter-electrode,7,8 and electrolyte.9,10 To date, the highest light-to-electric conversion efficiency of 15% was achieved by optimizing the semiconductor photoanode with an organic–inorganic hybrid perovskite absorber.11 For the most part, TiO2 semiconductors have been the metal oxide photoanode of choice for DSSCs due to their superior device efficiency resulting from their high specific surface area and relevant band gap position (3.0 eV).12However, the wide band gap semiconductor SnO2 (3.6 eV) has attracted much scientific attention due to its high electronic conductivity/mobility and good stability under long-term UV irradiation, which make it suitable as a photoanode material for DSSCs.13 Li and co-workers prepared branch-type SnO2 nanowires with efficient electron channels and excellent light scattering properties, and obtained a photovoltaic efficiency of 4.23% after TiCl4 post-treatment.14 Recently, Ramakrishna and co-workers developed SnO2 flower-shaped nanostructures using an electrospinning method and reported a photovoltaic efficiency of 3.0%, which was much higher than that reported for a SnO2 fiber system.15 Tremel and co-workers reported that the use of SnO2 nanostructures prepared via a microwave-assisted solvothermal process enhanced DSSC efficiency up to 3.16%.16 In addition, Toupance and co-workers reported an efficiency of 3.2% of a DSSC using tin dioxide nanoparticles of different sizes and shapes as a photoanode.17 However, there are only a few reports of solid-state DSSCs (ssDSSCs) with SnO2 nanostructures as a photoanode, presumably because of difficulties in generating large pores, which are important for pore filling by solid polymer electrolyte.18,19
In recent years, a great deal of research has focused on using semiconductor metal oxides as photocatalysts due to their potential applications in environmental remediation. A photocatalyst should have high photocatalytic activity with low toxicity and excellent photocatalytic stability. Like many semiconductor metal oxides, use of nanoscale SnO2 structures can potentially increase photocatalytic performance because these structures have a high specific surface area that facilitates efficient degradation of environmental pollutants, in addition to excellent stability as well as acid and alkali resistant properties. Xiong and coworkers reported that the photocatalytic activity of SnO2@CdS nanowire heterostructures increased by more than 109% relative to that of neat SnO2 nanowires.20 Furthermore, Zhu and co-workers suggested that the photocatalytic activities of α-Fe2O3@SnO2 core–shell shuttle-like nanocomposites were strongly dependent on the presence of core–shell heteronanostructures.21 SnO2, however, has only been used as a minor component of semiconductor composite photocatalysts. Hierarchical mesoporous nanostructures consisting of small particles and aggregated nanostructures can also potentially be used in photocatalytic devices. Small particles would provide a large surface area for photodegradation reactions, whereas a secondary aggregated nanostructure could potentially reflect incident photons to prolong their traveling distance, thereby improving light harvesting. A facile method to prepare hierarchical mesoporous SnO2 nanostructures with the desired characteristics is therefore critical if these nanostructures are to be used for practical photocatalytic applications.
One approach that can be taken to improve DSSC performance is to control the interface between the fluorine-doped tin oxide (FTO) substrate and the main semiconductor photoanode layer. Recently, Kim and co-workers demonstrated that the presence of a multi-functional, organized mesoporous SnO2 interfacial (om-SnO2 IF) layer improved light harvesting, enhanced electron transport, reduced recombination, and decreased interfacial/internal resistance compared to when this layer was not present.22 Lee and coworkers reported improved DSSC performance based on the presence of a nanoparticle-based ultrathin SnO2 layer as a buffer layer prepared using a layer-by-layer self-assembly technique.23 Solid electrolytes can also enhance the stability and photovoltaic performance of DSSCs. However, ssDSSCs fabricated with solid electrolyte typically perform less well than their liquid electrolyte counterparts. This is mostly due to insufficient penetration of the large molecular volume solid electrolyte into the less-organized particulate photoanode, resulting in increased electrode/electrolyte interfacial resistance.24 Hierarchical mesoporous nanostructures are promising ssDSSC photoanode candidates due to their inherently high porosity, ordered large pores, and good interconnectivity.
Mesoporous SnO2 nanostructures hundreds of nanometers in size that show monodispersity are of particular interest, because these nanostructures can simultaneously increase surface area, porosity, and adsorption, and have been shown to enhance the performance of gas sensors and lithium storage devices.25–27 Mesoporous SnO2 nanostructures are generally prepared using a hydrophilic homopolymer such as poly(vinyl pyrrolidone) or poly(ethylene glycol). However, a homopolymer functions as a compatibilizer in hydrothermal processes, rather than a structure-directing agent. Amphiphilic nanostructural copolymers such as block and graft copolymers are better candidates than hydrophilic homopolymers because Sn precursors would be selectively incorporated into hydrophilic domains during the hydrothermal reaction, resulting in formation of a hierarchical mesoporous structure. Although titania (TiO2) with a hierarchical mesoporous structure has been suggested for DSSC applications,18,19 hierarchical mesoporous SnO2 nanostructures with two pore sizes and large pores have not previously been reported.
In this work, thus, we synthesized hierarchical mesoporous SnO2 (HM-SnO2) spheres for photovoltaic and photocatalytic applications via a facile solvothermal reaction using graft copolymer as a structure-directing agent. Graft copolymer of poly(vinyl chloride)-g-poly(oxyethylene methacrylate) (PVC-g-POEM) was synthesized via atom transfer radical polymerization (ATRP). Graft copolymers are easier to synthesize and cost less than block copolymers. HM-SnO2 spheres were characterized by Field emission-scanning electron microscopy (FE-SEM), energy-filtered transmission electron microscopy (EF-TEM), X-ray diffraction (XRD), in addition to Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) measurements. An om-SnO2 IF layer was utilized as a buffer layer in ssDSSCs with a solid electrolyte, and the influence of the material structure on photovoltaic performance was also investigated. Photovoltaic and photocatalytic cells based on HM-SnO2 spheres were fabricated and characterized in detail by measuring reflectance and incident photon to current conversion efficiency (IPCE), by generating photocurrent density–voltage (J–V) curves, and by performing intensity-modulated photocurrent/voltage spectroscopy (IMPS/IMVS).
Experiment section
Materials
Poly(vinyl chloride) (PVC, Mw = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Mn = 55
000 g mol−1, Mn = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), poly(oxyethylene methacrylate) (POEM, poly(ethylene glycol) methyl ether methacrylate, Mn = 475 g mol−1), 1,1,4,7,10,10-hexamethyltriethylene tetramine (HMTETA, 99%), copper(I) chloride (CuCl, 99%), tin chloride pentahydrate (SnCl4·5H2O, 99%), tin(II) chloride (SnCl2, 99%), ethyl cellulose, α-terpineol, 1-butylimidazole, 4-chloromethylstyrene, lithium iodide (LiI), magnesium sulfate (MgSO4), 2,2′-azobisisobutyronitrile (AIBN), iodine (I2), 3-methoxypropionitrile, butylmethylimidazolium iodide, guanidinium thiocyanate, 4-tertbutylpyridine, valeronitrile, chloroplatinic acid hexahydrate (H2PtCl6), and methyl orange were purchased from Sigma-Aldrich (St. Louis, MO). Tetrahydrofuran (THF), N-methyl pyrrolidone (NMP), methanol, 2-propanol, chloroform, acetonitrile, diethylether, and ethyl acetate were purchased from J. T. Baker. Deionized water (>18 MΩ m) was obtained with a Millipore water purification system. Ruthenium dye (535-bisTBA, N719) and 60 μm-thick Surlyn were purchased from Solaronix, Switzerland. Fluorine-doped tin oxide (FTO) conducting glass substrate (TEC8, 8 ohms sq−1, 2.3 mm-thick) was purchased from Pilkington, France. All chemicals and solvents reagents were of analytical grade and were used as-received without further purification.
000 g mol−1), poly(oxyethylene methacrylate) (POEM, poly(ethylene glycol) methyl ether methacrylate, Mn = 475 g mol−1), 1,1,4,7,10,10-hexamethyltriethylene tetramine (HMTETA, 99%), copper(I) chloride (CuCl, 99%), tin chloride pentahydrate (SnCl4·5H2O, 99%), tin(II) chloride (SnCl2, 99%), ethyl cellulose, α-terpineol, 1-butylimidazole, 4-chloromethylstyrene, lithium iodide (LiI), magnesium sulfate (MgSO4), 2,2′-azobisisobutyronitrile (AIBN), iodine (I2), 3-methoxypropionitrile, butylmethylimidazolium iodide, guanidinium thiocyanate, 4-tertbutylpyridine, valeronitrile, chloroplatinic acid hexahydrate (H2PtCl6), and methyl orange were purchased from Sigma-Aldrich (St. Louis, MO). Tetrahydrofuran (THF), N-methyl pyrrolidone (NMP), methanol, 2-propanol, chloroform, acetonitrile, diethylether, and ethyl acetate were purchased from J. T. Baker. Deionized water (>18 MΩ m) was obtained with a Millipore water purification system. Ruthenium dye (535-bisTBA, N719) and 60 μm-thick Surlyn were purchased from Solaronix, Switzerland. Fluorine-doped tin oxide (FTO) conducting glass substrate (TEC8, 8 ohms sq−1, 2.3 mm-thick) was purchased from Pilkington, France. All chemicals and solvents reagents were of analytical grade and were used as-received without further purification.
Preparation of HM-SnO2 spheres using graft copolymer
HM-SnO2 spheres were prepared by the solverthermal method using tin chloride pentahydrate as a precursor and PVC-g-POEM graft copolymer as a structure-directing agent. PVC-g-POEM graft copolymer consisting of a poly(vinyl chloride) backbone and poly(oxyethylene methacrylate) side chains was synthesized via atomic transfer radical polymerization (ATRP) according to a previously reported method.22,28 Typically, 0.2 g graft copolymer was dissolved in 36 mL of THF. Separately, 0.15 g of tin chloride pentahydrate was dissolved and stabilized in the mixture solution of 4 mL H2O with vigorous stirring. After aging for 15 min, SnO2 precursor solution was added to the graft copolymer solution. After vigorous stirring for 1 h, the solution was transferred to an autoclave and kept at 180 °C for 3 h and then, the autoclave was cooled naturally in air. The resultant precipitate was centrifuged and washed with THF and ethanol three times and then dried at room temperature for further experiments and characterization. Samples were then calcined at 500 °C for 2 h in air to remove residual organic components. In addition, NP-SnO2 spheres were made from tin chloride pentahydrate without graft copolymer as a control.Preparation of HM-SnO2 sphere photoanodes
Before deposition of the HM-SnO2 sphere layer, clean FTO glass substrates were coated with a multi-functional om-SnO2 IF layer solution based on a graft copolymer template, followed by calcination at 450 °C for 30 min.22 Then, SnO2 paste (HM-SnO2 spheres or NP-SnO2 spheres) was deposited on the om-SnO2 IF layer-coated FTO glass using a doctor-blade technique and successive annealing at 450 °C for 30 min. To prepare a screen-printable viscous SnO2 paste, 0.1 g of HM-SnO2 spheres or NP-SnO2 spheres was dispersed in 2 mL of ethanol with sonication for 30 min, followed by stirring for 1 h. Then, 0.5 mL of ethyl cellulose solution dissolved in ethanol at 10 wt% and 0.4 g of α-terpineol were added to the SnO2 suspension and stirred at room temperature for 20 min, followed by sonication for 30 min. The paste was concentrated by evaporation of ethanol at room temperature for 1 day. Then, HM-SnO2 sphere and NP-SnO2 sphere photoanodes were sensitized with ruthenium solution (10−4 M) in ethanol at 50 °C for 2 h in a dark room without any post-treatment. Finally, the dye-sensitized solar cell photoanodes were immersed in absolute ethanol for 5 min to remove non-adsorbed dye on the surface.Fabrication of DSSCs
Two types of electrolytes were used to fabricate DSSCs: (1) solid-state polymerized ionic liquid (PIL), i.e., poly(1-((4-ethenylphenyl)methyl)-3-butyl-imidazolium iodide) (PEBII) and (2) liquid electrolyte consisting of 1-butyl-3-methylimidazolium iodide, iodine (I2), guanidinium thiocyanate, and 4-tert-butylpyridine (TBP) in acetonitrile and valeronitrile. For the ssDSSC system, PIL electrolyte solution was prepared by dissolving a PEBII solution (2 and 10 wt%) in acetonitrile. The PIL electrolyte solution infiltrated deeply into the photoanode and covered the counter electrode.24 Cells were placed in a drying oven at 40 °C for 24 h and subsequently in a vacuum oven at 40 °C for 24 h to ensure complete solvent evaporation. Then, ssDSSCs were placed in a vacuum oven for a day to permit complete evaporation of the solvent and then sealed with an epoxy resin. In the case of the liquid system, DSSCs with liquid electrolyte were assembled using 60 μm-thick Surlyn, then the inner spaces of the DSSCs were filled with a liquid electrolyte solution through a drilled hole on the counter electrode. Liquid electrolyte solution comprised 0.6 M 1-butyl-3-methylimidazolium iodide, 0.03 M I2, 0.1 M guanidinium thiocyanate, and 0.5 M 4-tert-butylpyridine in a mixture of acetonitrile and valeronitrile (v/v, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). Pt-coated counter electrodes were prepared by spin coating of a 7 mM H2PtCl6 solution in isopropyl alcohol and calcination at 450 °C for 30 min. The photoanode active area, determined by the aperture of a black mask, was 0.16 cm2.
15). Pt-coated counter electrodes were prepared by spin coating of a 7 mM H2PtCl6 solution in isopropyl alcohol and calcination at 450 °C for 30 min. The photoanode active area, determined by the aperture of a black mask, was 0.16 cm2.
Measurement of photocatalytic activity
In a typical reaction, 0.005 g of prepared photocatalyst (HM-SnO2 spheres, NP-SnO2 spheres) was added to 500 mL of methyl orange (MO) aqueous solution (20 mg L−1) in a quartz reactor with a sealed bottom. Prior to irradiation, the solution was stirred for 30 min in the dark to stabilize and equilibrate adsorption of MO on the surface of the photocatalyst. The solution was irradiated with UV light with constant stirring to ensure homogeneity of the photocatalyst in the solution. Concentration of MO was determined using UV-Vis absorption spectra and the maximal absorbance peak value (at 462.5 nm) was used to determine the amount of degraded MO, corresponding to the photo-activity of the catalyst. Residual MO content was calculated as C/Co, where C and Co are the concentrations of the tested solution.Characterization
Morphologies, sizes, and intrinsic structures of HM-SnO2 and NP-SnO2 spheres were determined by FE-SEM (SUPRA 55VP, Germany, Carl Zeiss) and EF-TEM (LIBRA 120). Crystalline structures of HM-SnO2 and NP-SnO2 spheres were analyzed using an X-ray diffraction (XRD, Rigaku) 18 kW rotating anode X-ray generator equipped with CuKα radiation (λ = 1.5406 Å). Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) measurements were conducted using a using a BELSORP-mini II with N2 as the adsorbate at liquid nitrogen temperature. In preparation for XRD measurements, TiO2 films were degassed at 70 °C under dynamic vacuum (10−2 Torr) for 1 h. Diffused reflectance spectra of HM-SnO2 and NP-SnO2 spheres were collected in the wavelength range of 400–800 nm using a UV-visible spectrophotometer (Hewlett-Packard, Hayward, CF). IPCE system for DSSCs (K3100) was used to provide incident light with a wavelength range of 400 to 800 nm for incident photon to current conversion efficiency (IPCE) measurements. Electrochemical impedance spectroscopy (EIS) analysis was used to investigate the internal resistance at the electrode/electrolyte interface and the recombination kinetics of photoanode in DSSCs. Photoanode thickness was measured using an alpha-step IQ surface profile (KLA Tencor). Photocurrent density–voltage curves (J–V) were generated using a Keithley 2400 source meter under 1 sun illumination (AM 1.5G; 100 mW cm−2) with a solar light simulator (Oriel, 91193). A NREL-calibrated Si cell with an optical filter was used to adjust the light intensity of the 1000 W xenon arc lamp that served as the light source. During photocurrent density–voltage measurements, DSSCs were covered with a black mask with an aperture to avoid additional light coming through the lateral space. Photoelectrochemical performance was evaluated using the following equations:| FF = Vmax × Jmax/Voc × Jsc | (1) |
| η = Vmax × Jmax/Pin × 100 = Voc × Jsc × FF/Pin × 100 | (2) |
Results and discussion
Synthesis and characterization of HM-SnO2 spheres
To synthesize HM-SnO2 spheres with two pore sizes, we used PVC-g-POEM graft copolymer as a structure-directing agent; this copolymer can be easily synthesized via ATRP (Scheme 1). ATRP creates nanometer-scale organic–inorganic hybrid materials through self-assembly of graft copolymer copolymers.28 During a solverthermal process at 180 °C for 3 h, a hydrophilic Sn precursor, i.e. tin chloride pentahydrate, preferentially interacts and embeds into hydrophilic POEM domains of microphase-separated PVC-g-POEM graft copolymer. These hybrids can subsequently be transformed into nanostructured tin oxides with mesopores by calcination of the blend. Thus, graft copolymer not only inhibits the aggregation of SnO2 nanocrystals during the solverthermal process, but also generates mesopores with controlled porosity after calcination at 500 °C.To improve the photovoltaic and photocatalytic performance of a photoanode, it should have a high specific surface area, good light scattering ability, excellent connectivity between nanoparticles, and sufficient electrolyte infiltration into electrode pores. In this context, we performed EF-TEM and FE-SEM measurements to further characterize the structure and morphology of HM-SnO2 spheres. NP-SnO2 spheres without graft copolymer were also assessed for structural comparison. Representative EF-TEM and FE-SEM images of the as-synthesized NP-SnO2 spheres are shown in Fig. 1a and c; spheres were relatively smooth with an average size of 100–300 nm, and had a nonporous morphology. Formation of spherical structures is an interesting result of the solverthermal process, but NP-SnO2 spheres are not appropriate for photovoltaic and photocatalytic applications due to their low surface area resulting from their lack of pores. Incorporation of the graft copolymer into the Sn precursor solution yielded HM-SnO2 spheres with higher porosity, a larger surface area, and better interconnectivity than the NP-SnO2 spheres (Fig. 1b and d). Secondary colloidal spheres with a diameter between 100–300 nm formed; these consisted of numerous primary nanoparticles of 15 nm in diameter, which could enhance light scattering. These results indicated that PVC-g-POEM graft copolymer played a significant role as a structure-directing agent to control the agglomeration of Sn precursor during the solverthermal process. Well-interconnected, porous HM-SnO2 structures consisting of small particles aggregated into larger clusters were obtained; we expected this combination of small particles and larger clusters to result in a synergistic improvement in light harvesting, electron transport, electrolyte penetration, and consequently photovoltaic and photocatalytic performance.
 | ||
| Fig. 1 EF-TEM images of (a) NP-SnO2 spheres, (b) HM-SnO2 spheres and (c) FE-SEM images of NP-SnO2 spheres, (d) HM-SnO2 spheres. | ||
Phase structure and crystallite size of nanoscale metal oxides have a significant effect on photovoltaic and photocatalytic performance. Thus, we characterized NP-SnO2 and HM-SnO2 spheres by X-ray diffraction; results are shown in Fig. 2. Crystallite size was estimated using the following Scherrer formula:
D = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (3) |
Performance of photovoltaic and photocatalytic applications is strongly dependent on both the surface area and porosity of nanoscale metal oxides. Thus, N2 absorption–desorption measurements were taken to investigate the surface area and porosity of NP-SnO2 and HM-SnO2 spheres, as shown in Fig. 3. Specific surface area was calculated using the BET method, while average pore diameter was determined using the BJH method. Specific surface area of NP-SnO2 spheres was low at 37.2 m2 g−1. Based on the BJH pore size distribution curves, we concluded that pores with a size of 100–200 nm were dominant in NP-SnO2 spheres, consistent with the FE-SEM and TEM results. Specific surface area of HM-SnO2 spheres was determined to be 85.3 m2 g−1, which is approximately 2.3-fold higher than that of the NP-SnO2 spheres. In addition, HM-SnO2 spheres exhibited a Type IV N2 isotherm loop (according to the IUPAC classification) where the hysteresis loop approached relatively higher pressures, indicating the presence of an interconnected mesoporous structure. Particularly, tailing upward at a higher hysteresis loop relative pressure (i.e. P/P0 = 1) indicated the formation of a macroporous structure, with the contribution of large pores beyond the mesopore scale. HM-SnO2 spheres showed a remarkable bimodal pore structure with mesopores 23.5 nm in diameter and macropores 162.3 nm in diameter. This indicated that PVC-g-POEM graft copolymer controlled the agglomeration of Sn precursor as a structure-directing agent. It should be noted that the specific surface area of commercially available nanocrystalline SnO2 is 23.1 m2 g−1, which is much smaller than that of the HM-SnO2 spheres.31 Furthermore, the specific surface area of the HM-SnO2 spheres (85.3 m2 g−1) was larger than those of previously reported SnO2 mesoporous nanostructures (78.2 m2 g−1 and 43 m2 g−1).25–27 We predicted that these HM-SnO2 spheres would promote greater generation of photoelectrons, allow facile transport of the electrolyte, and enhance photovoltaic and photocatalytic device performance compared to previously reported SnO2 mesoporous nanostructures.
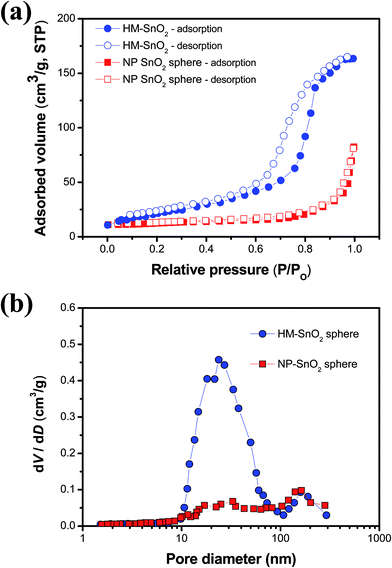 | ||
| Fig. 3 (a) BET N2 adsorption–desorption isotherms and (b) BJH pore size distribution curves of NP-SnO2 spheres and HM-SnO2. | ||
Photovoltaic performance of DSSCs
Because the performance of photovoltaic applications is strongly dependent on light-scattering by photoanodes, we measured the UV-visible reflectance spectra of an as-prepared HM-SnO2 sphere layer with and without N719 dye sensitizer, as shown in Fig. 4a and b. We used a NP-SnO2 sphere layer as a control. We also used a multi-functional om-SnO2 IF layer as a buffer layer because this structure has been previously shown to enhance light harvesting, improve electron transport by suppression of recombination or back-reactions, and reduce interfacial/internal resistance, yielding high-performance DSSCs with good long-term stability.22 As observed in the plan-view FE-SEM image (Fig. S1a†), the om-SnO2 IF layer consisted of tightly adhered nanoparticles without any structural defects and showed excellent interconnectivity and well-ordered porosity. The om-SnO2 IF layer was approximately 500 nm-thick, and adhered tightly to the FTO glass with good interfacial contacts (Fig. S1b†). As shown in Fig. 4a, high reflectance values above 50% were observed for both HM-SnO2 and NP-SnO2 spheres layer without N719 dye sensitizer in the visible light wavelength range of 400–800 nm, indicating excellent light-scattering ability. It should be noted that the less-organized, commercially available nanocrystalline SnO2 photoanode showed a reflectance value below 20% over the entire wavelength regions (Fig. S2†). On the other hands, after N719 dye sensitizer adsorption, the reflectance of the HM-SnO2 sphere layer was reduced to 20% in the 400–500 nm wavelength region, which is much lower than that of the NP-SnO2 sphere layer (∼40%), as shown in Fig. 4b. These results can be explained by the fact that the presence of aggregated, large nanostructures improved light scattering, leading to an increase in red light harvesting. Primary small nanoparticles in HM-SnO2 spheres provided a large specific surface area for dye adsorption; we therefore also expected to observe an increase in the short circuit current (Jsc). | ||
| Fig. 4 Reflectance spectra of a NP-SnO2 sphere layer and HM-SnO2 sphere layer on multi-functional om-SnO2 IF layer-coated FTO glass (a) without and (b) with N719 dye sensitizer. | ||
IPCE is defined as the ratio of the number of electrons in the external circuit produced by an incident photon at a given wavelength. Therefore, light-harvesting ability can be evaluated by measuring the IPCE spectra of a device. IPCE values of ssDSSCs fabricated with NP-SnO2 and HM-SnO2 spheres on a multi-functional om-SnO2 IF layer are shown in Fig. 5 as a function of illumination wavelength. PEBII was synthesized via free radical polymerization and employed as a solid electrolyte. This obviated the need for incorporation of additives such as I2, ionic liquids, and salts.32 ssDSSCs fabricated with HM-SnO2 on a multi-functional om-SnO2 IF layer had significantly higher absolute IPCE values than ssDSSCs fabricated with NP-SnO2 sphere cells over the whole spectral range, which we attributed to the superior light harvesting ability of HM-SnO2 spheres (Fig. 5a). Light-scattering effect was greater for longer wavelengths (>520 nm) because more light was transmitted through the photoanode and scattered in this region.33 Spectrally selective enhancement for these two systems was considerable, as shown in Fig. 5b. Notably, the normalized IPCE value of ssDSSCs based on the HM-SnO2 sphere system showed a significant broadening below 520 nm as compared with the NP-SnO2 sphere system. Such broadening of the spectral domain indicated improved light harvesting in this wavelength region, which we attributed to enhanced dye loading due to the large specific surface area of the HM-SnO2 system, which increased photoelectrical response. The high IPCE value of ssDSSCs based on HM-SnO2 spheres indicated that these ssDSSCs had superior light reflectance and dye loading characteristics than ssDSSCs based on NP-SnO2 spheres.
The mechanism underlying the photovoltaic performance improvement in the HM-SnO2 spheres on a multi-functional om-SnO2 IF layer based ssDSSCs was further investigated by measuring the recombination resistance (Rrec) and electron lifetime (τn) of the devices as a function of bias voltage as shown in Fig. 6. The electron lifetime (τn) was derived from the product between the resistance of recombination and the capacitance for the electron transfer. Larger recombination resistance and longer electron lifetime were obtained in the HM-SnO2 spheres on a multi-functional om-SnO2 IF layer based ssDSSCs, indicating increased electron transport and reduced interfacial charge recombination loss. It indicates the importance of an organized HM-SnO2 spheres structure for electron transport, which is attributed to the good interconnectivity resulting in the improved interfacial contact of the electrode/electrolyte. And, our suggestions about the relationship between hierarchical organized structure and electron transport are consistent with a previous study by the Jiang group.34
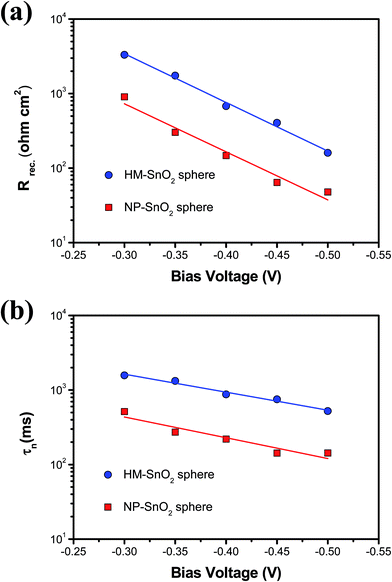 | ||
| Fig. 6 (a) Recombination resistance (Rrec) and (b) electron lifetime (τn) of ssDSSCs fabricated with NP-SnO2 spheres and HM-SnO2 spheres on a multi-functional om-SnO2 IF layer photoanode. | ||
We next investigated the performance of ssDSSCs with HM-SnO2 spheres on a multi-functional om-SnO2 IF layer as photoanode and solid-state electrolyte. Photocurrent density–voltage (J–V) characteristics are shown in Fig. 7. Table 1 summarizes photovoltaic parameters such as Voc, Jsc, fill factor (FF), and light-to-electricity conversion efficiency (η). In addition, reference devices were fabricated using NP-SnO2 spheres on a multi-functional om-SnO2 IF layer as the photoanode material. As expected, Voc and Jsc values for ssDSSCs with HM-SnO2 spheres were much higher than those for ssDSSCs based on NP-SnO2 spheres. Notably, devices with photoanodes comprising HM-SnO2 spheres on a multi-functional om-SnO2 IF layer had a Voc of 0.56 V, Jsc of 10.7 mA cm−2, and FF of 0.57, resulting in an η of 3.4%, which is much higher than the η of the NP-SnO2 sphere system (1.9%). This value is one of the highest reported for SnO2-based ssDSSCs employing a solid-state electrolyte and N719 ruthenium dye as the sensitizer.
| Photoanode | Electrolyte | Voc (V) | Jsc (mA cm−2) | FF | η (%) |
|---|---|---|---|---|---|
| a Solid electrolyte: poly(1-((4-ethenylphenyl)methyl)-3-butyl-imidazolium iodide) (PEBII).b Liquid electrolyte: 1-butyl-3-methylimidazolium iodide, I2, guanidinium thiocyanate, and 4-tert-butylpyridine in a mixture of acetonitrile and valeronitrile. | |||||
| NP-SnO2 spheres/om-SnO2 IF layer | Solid | 0.52 | 6.3 | 0.56 | 1.9 |
| Liquid | 0.49 | 7.8 | 0.58 | 2.2 | |
| HM-SnO2 spheres/om-SnO2 IF layer | Solid | 0.56 | 10.7 | 0.57 | 3.4 |
| Liquid | 0.52 | 12.3 | 0.58 | 3.7 | |
One possible reason for the enhanced Voc value is suppression of charge recombination and electron back-reactions in the well-interconnected HM-SnO2 sphere system. Voc is strongly dependent on recombination or back-reactions, and a higher Voc value can be attained by suppressing those reactions as reflected by the following equation:
| Voc = (RT/nF) × ln(AI/(n0k1(I3−) + n0k2(D+))) | (4) |
| Jsc = q × ηlh × ηinj × ηcol × I0 | (5) |
DSSCs were also fabricated with a liquid state electrolyte containing iodine (I2), 1-butyl-3-methylimidazolium iodide, guanidinium thiocyanate, and 4-tert-butylpyridine in a mixture of acetonitrile and valeronitrile. J–V curves for these DSSCs are shown in Fig. S3† and their photovoltaic parameters are summarized in Table 1. A Jsc of 12.3 mA cm−2, Voc of 0.58 V, FF of 0.58, and a η of 3.7% were obtained for DSSCs based on HM-SnO2 spheres and a multi-functional om-SnO2 IF layer under 1 sun illumination (AM 1.5G; 100 mW cm−2). This efficiency is high compared to that of DSSCs with a SnO2 photoanode and liquid state electrolyte.17,37
ssDSSCs fabricated without a multi-functional om-SnO2 IF layer exhibited an efficiency of only 1.7% at 100 mW cm−2, as shown in Fig. 7b and Table 2. This result again indicates the importance of an anti-reflective, cascadal energy band gap, good interconnectivity, and high electrical conductivity.22 We also measured the long-term stability of ssDSSCs comprising HM-SnO2 spheres on a multi-functional om-SnO2 IF layer photoanode with solid state electrolyte; results are shown in Fig. S4.† Energy conversion efficiency of these ssDSSCs decreased very slowly, and efficiency remained close to the initial value even up to 30 days. This implies that our SnO2-based device with solid-state electrolyte has a stable dye/photoanode/electrolyte interface, resulting in significantly enhanced long-term stability.
| Photoanode | Voc (V) | Jsc (mA cm−2) | FF | η (%) |
|---|---|---|---|---|
| HM-SnO2 spheres without an om-SnO2 IF layer | 0.48 | 8.5 | 0.42 | 1.7 |
| HM-SnO2 spheres with an om-SnO2 IF layer | 0.56 | 10.7 | 0.57 | 3.4 |
Photocatalytic performance
To evaluate the photocatalytic performance of the HM-SnO2 spheres, we evaluated the oxidation of methyl orange under UV light irradiation. For comparison, we also evaluated the photocatalytic performance of NP-SnO2 spheres. Before UV light illumination, colloidal suspensions of the two types of SnO2 spheres and methyl orange were stirred in the dark for 30 min to ensure that organic pollutants was adsorbed to saturation on the inner and outer surfaces of the catalyst. NP-SnO2 spheres were not able to fully degrade the methyl orange molecules after 60 min of UV light irradiation, as shown in Fig. 8a. However, methyl orange was completely decomposed in the presence of HM-SnO2 spheres after only 40 min of irradiation with UV light. It should be noted that HM-SnO2 spheres showed much greater activity than Degussa P25 (see Fig. S5†). We attributed the superior photocatalytic performance of HM-SnO2 spheres to the presence of secondary aggregated nanostructures composed of primary small nanoparticles with a high surface area, which favored efficient light harvesting via enhanced methyl orange molecule loading and good light reflection. Their pseudo-first-order kinetic rate plots in Fig. S6† indicate that HM-SnO2 spheres have the excellent enhanced photocatalytic activity. In addition, we performed a reutilization experiment to evaluate the stability of HM-SnO2 spheres as photocatalysts. When the photocatalytic experiment was completed, the residue was centrifuged and placed in an oven at 50 °C for a day. Then, the residue was dispersed in methyl orange solution and the photocatalytic experiment was repeated. As shown in Fig. 8b, there was only a slight decrease in the photocatalytic performance of HM-SnO2 spheres after five cycles, demonstrating that these spheres have good photocatalytic stability. | ||
| Fig. 8 (a) Photocatalytic reactions of NP-SnO2 spheres and HM-SnO2 spheres with methyl orange under UV irradiation, (b) photocatalytic recycling test of HM-SnO2 spheres. | ||
Conclusions
In summary, we prepared HM-SnO2 spheres by solvothermal reaction with PVC-g-POEM graft copolymer as a structure-directing agent and investigated their photovoltaic and photocatalytic performance. om-SnO2 IF layer was used a buffer layer between the HM-SnO2 spheres and the FTO substrates in DSSCs to improve light harvesting, enhance electron transport, decrease charge recombination, and reduce interfacial/internal resistance. ssDSSCs fabricated with a photoanode comprising HM-SnO2 spheres on an om-SnO2 IF layer with the solid electrolyte of PEBII exhibited an energy conversion efficiency of 3.4% at 100 mW cm−2, which was much higher than that attained with the NP-SnO2 sphere system (1.9%). We attributed the outstanding improvement in photovoltaic performance to an increase in specific surface area, improved light scattering, and excellent pore filling by the solid-state electrolyte. Higher specific surface area and better light scattering ability of HM-SnO2 spheres compared to NP-SnO2 spheres contributed to the enhanced photocatalytic activity and good recycling performance of the former. These results can guide future designs of hierarchical mesoporous structures to realize further photovoltaic and photocatalytic improvements.Acknowledgements
This work was supported by the Korea Center for Artificial Photosynthesis (KCAP) (2009-0093883), the Energy Efficiency & Resources of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) (20122010100040) and the Converged Energy Materials Research Center program of Defense Acquisition Program Administration and Agency for Defense Development.References
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737 CrossRef.
- J. Liang, G. Zhang, H. Xia and W. Sun, RSC Adv., 2014, 4, 12649 RSC.
- Y. Xiao, J. Wu, G. Yue, J. Lin, M. Huang, L. Fan and Z. Lan, RSC Adv., 2012, 2, 10550 RSC.
- L. Chen, Y. Zhou, H. Dai, Z. Li, T. Yu, J. Liu and Z. Zou, J. Mater. Chem. A, 2013, 1, 11790 CAS.
- S. Cai, G. Tian, X. Li, J. Su and H. Tian, J. Mater. Chem. A, 2013, 1, 11295 CAS.
- G. D. Sharma, D. Daphnomili, K. S. V. Gupta, T. Gayathri, S. P. Singh, P. A. Angaridis, T. N. Kitsopoulos, D. Tasis and A. G. Coutsolelos, RSC Adv., 2013, 3, 22412 RSC.
- K. Yu, Z. Wen, H. Pu, G. Lu, Z. Bo, H. Kim, Y. Qian, E. Andrew, S. Mao and J. Chen, J. Mater. Chem. A, 2013, 1, 188 CAS.
- P.-W. Chen, C.-P. Lee, L.-Y. Chang, J. Chang, M.-H. Yeh, L.-Y. Lin, R. Vittal, J.-J. Lin and K.-C. Ho, RSC Adv., 2013, 3, 5871 RSC.
- Y.-F. Chan, C.-C. Wang and C.-Y. Chen, J. Mater. Chem. A, 2013, 1, 5479 CAS.
- F. Bella, J. R. Nair and C. Gerbaldi, RSC Adv., 2013, 3, 15993 RSC.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Gratzel, Nature, 2013, 499, 316 CrossRef CAS PubMed.
- H.-Z. Chen, Y.-Y. Zhang, X. Gong and H. Xiang, J. Phys. Chem. C, 2014, 118, 2333 CAS.
- H. J. Snaith and C. Ducati, Nano Lett., 2010, 10, 1259 CrossRef CAS PubMed.
- X. Hou, Y. Hu, H. Jiang, J. Huo, Y. Li and C. Li, J. Mater. Chem. A, 2013, 1, 13814 CAS.
- E. N. Kumar, R. Jose, P. S. Archana, C. Vijila, M. M. Yusoff and S. Ramakrishna, Energy Environ. Sci., 2012, 5, 5401 CAS.
- A. Birkel, Y.-G. Lee, D. Koll, X. V. Meerbeek, S. Frank, M. J. Choi, Y. S. Kang, K. Char and W. Tremel, Energy Environ. Sci., 2012, 5, 5392 CAS.
- L. Cojocaru, C. Olivier, T. Toupance, E. Sellier and L. Hirsch, J. Mater. Chem. A, 2013, 1, 13789 CAS.
- W.-Y. Cheng, J. R. Deka, Y.-C. Chiang, A. Rogeau and S.-Y. Lu, Chem. Mater., 2012, 24, 3255 CrossRef CAS.
- Z. Li, Y. Zhou, G. Xue, T. Yu, J. Liu and Z. Zou, J. Mater. Chem., 2012, 22, 14341 RSC.
- J. Pan, J. Li, Z. Yan, B. Zhou, H. Wu and X. Xiong, Nanoscale, 2013, 5, 3022 RSC.
- L.-P. Zhu, N.-C. Bing, D.-D. Yang, Y. Yang, G.-H. Liao and L.-J. Wang, CrystEngComm, 2011, 13, 4486 RSC.
- J. T. Park, S. H. Ahn, D. K. Roh, C. S. Lee and J. H. Kim, ChemSusChem, 2014 DOI:10.1002/cssc.201301215.
- Y. J. Kim, K. H. Kim, P. Kang, H. J. Kim, Y. S. Choi and W. I. Lee, Langmuir, 2012, 28, 10620 CrossRef CAS PubMed.
- J. T. Park, W. S. Chi, D. K. Roh, S. H. Ahn and J. H. Kim, Adv. Funct. Mater., 2013, 23, 26 CrossRef CAS.
- X. Wang, S. Qiu, J. Liu, C. He, G. Lu and W. Liu, Eur. J. Inorg. Chem., 2014, 2014, 863 CrossRef CAS.
- X. Wang, S. Qiu, C. He, G. Lu, W. Liu and J. Liu, RSC Adv., 2013, 3, 19002 RSC.
- X. Yin, L. Chen, C. Li, Q. Hao, S. Liu, Q. Li, E. Zhang and T. Wang, Electrochim. Acta, 2011, 56, 2358 CrossRef CAS PubMed.
- D. K. Roh, S. J. Kim, H. Jeon and J. H. Kim, ACS Appl. Mater. Interfaces, 2013, 5, 6615 CAS.
- K. Zhu, N. R. Neale, A. Miedaner and A. J. Frank, Nano Lett., 2007, 7, 69 CrossRef CAS PubMed.
- ICDD-JCPDS database, no. 41-1445.
- J. Qian, P. Liu, Y. Xiao, Y. Jiang, Y. Cao, X. Ai and H. Yang, Adv. Mat., 2009, 21, 3663 CrossRef CAS.
- J. T. Park, J. H. Prosser, S. H. Ahn, S. J. Kim, J. H. Kim and D. Lee, Adv. Funct. Mater., 2013, 23, 2193 CrossRef CAS.
- J. T. Park, D. K. Roh, R. Patel, E. Kim, D. Y. Ryu and J. H. Kim, J. Mater. Chem., 2010, 20, 8521 RSC.
- D. Wu, Z. Gao, F. Xu, J. Chang, W. Tao, J. He, S. Gao and K. Jiang, CrystEngComm, 2013, 15, 1210 RSC.
- J. T. Park, W. S. Chi, H. Jeon and J. H. Kim, Nanoscale, 2014, 6, 2718 RSC.
- P. Tiwana, P. Docampo, M. B. Johnston, H. J. Snaith and L. M. Herz, ACS nano, 2011, 5, 5158 CrossRef CAS PubMed.
- Z. Li, Y. Zhou, J. Song, T. Yu, J. Liu and Z. Zou, J. Mater. Chem. A, 2013, 1, 524 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03949a |
| This journal is © The Royal Society of Chemistry 2014 |




