Light harvesting of CdSe/CdS quantum dots coated with β-cyclodextrin based host–guest species through resonant energy transfer from the guests†
Francesca Villafiorita-Monteoleone*a,
Valentina Daitab,
Claudio Quartic,
Dario Perdicchiab,
Paola Del Butterob,
Guido Scaviaa,
Mirella del Zoppoc and
Chiara Botta*a
aIstituto per lo Studio delle Macromolecole, CNR, Via Bassini 15, 20133 Milano, Italy. E-mail: chiara.botta@ismac.cnr.it; Fax: +39 02 70636400; Tel: +39 02 23699734
bUniversity of Milano, Dipartimento di Chimica, Via Golgi 19, 20133, Milano, Italy
cPolitecnico di Milano Dipartimento di Chimica, Materiali e Ing. Chimica CMIC “G. Natta”, p.za Leonardo da Vinci 32, 20133, Milano, Italy
First published on 18th June 2014
Abstract
Films of nano-hybrids based on red emitting CdSe/CdS QDs functionalized with perthiolated β-cyclodextrin hosting a green emitting nitrobenzoxadiazole derivative show emission harvested by the host–guest organic system through resonant energy transfer from the organic host–guest species to the QD.
1. Introduction
Core–shell semiconductor Quantum Dots (QDs) are becoming valuable alternatives to organic dyes for many fluorescence-based applications, owing to their unique photochemical and photophysical properties, which include high photoluminescence (PL) Quantum Yields (QY), long PL lifetimes, large extinction coefficients, good chemical and photostability, broad absorption spectra with large Stokes shifts, and size-tunable PL emission spectra.1,2 Moreover, the possibility to add functionality to the QD surface, through the realization of a coating layer formed by host molecules, makes these materials ideal for the realization of hybrid host–guest systems.3,4β-cyclodextrin (CD) is an organic host well known for its ability to form inclusion complexes with various guest molecules of hydrophobic nature and suitable size thanks to its special molecular structure, composed by a hydrophobic internal cavity and a hydrophilic external surface.5,6 CD modified QDs (CD-QDs) have received much attention as fluorescence sensors. In fact CD-QDs emission properties are strongly modified by the non-covalent interactions between the surface-anchored CD host and the molecular guest, providing a powerful molecular recognition system.3,7,8
QD emission properties are modified either by Resonant Energy Transfer (RET) or by charge transfer (CT) processes occurring with molecules or with other QDs.8,9 While RET processes are extremely efficient in the down-conversion of the excitation,10,11 providing a color change in the emission, CT prevents radiative recombination of the e–h pair in the QD by inducing its dissociation and therefore act as an efficient PL quencher.12–14 Electron and energy transfer processes can therefore be designed to switch the luminescence of QDs in response to molecular recognition events, providing extremely sensitive probes15 or to efficiently sensitize the electrodes for solar cell applications.16 QDs can serve both as donors or acceptors when engaged in RET processes with chromophores.10 However, the need of good and stable emitters in the red or NIR region combined with the broad absorption spectra and high extinction coefficient of the QDs make them ideal as acceptors. Nevertheless, so far, while numerous papers report the use of QDs (mainly CdSe/CdS or CdSe/ZnS) as donors,11,17–19 few studies have demonstrated the use of QDs (mainly CdSe/CdZn) as acceptors.20–22 Thanks to their strong and size-dependent photoluminescence CdSe/CdS core–shell QDs have recently been shown to be ideal candidates for the fabrication of photoswitchable and electroluminescent devices.23,24 Therefore the design of donor–acceptor nano-hybrids where CdSe/CdS QDs have the function of acceptors are highly desirable for optoelectronic applications.
Here we present a new host–guest hybrid system QD/CD(3)/NBD(1), see Scheme 1, based on red emitting CdSe/CdS core–shell QDs functionalized with perthiolated β-cyclodextrin CD(3). A green emitting nitrobenzoxadiazole derivative NBD(1) molecule has been inserted in the CD cavity.
 | ||
| Scheme 1 Schematic illustration of the synthesis of the hybrid donor–acceptor system QD/CD(3)/NBD(1) (top) and structure of NBD(1) (bottom). | ||
We show that this hybrid system realizes an ideal nanoscale RET donor–acceptor dyad, where the organic molecule and the QD act as donor and acceptor, respectively. The CD host, besides stabilizing the QDs and avoiding their aggregation, has the important function to keep the donor molecules at a fixed distance from the QDs, optimal for efficient RET.
2. Experiment
2.1 General
Starting materials and solvents of analytical grade were obtained from commercial sources and used without further purification. All reagents were purchased from Sigma-Aldrich. Cyclodextrin was dried under vacuum over P2O5 prior use. CdSe/CdS core–shell QDs (λem = 625 nm) dispersed in hexane were obtained from Strem Chemicals.1H NMR (300 MHz) was taken with a Bruker AMX 300 instrument. Chemical shifts are given as parts per million from tetramethylsilane. Coupling constants (J) values are given in hertz and are quoted to ±0.1 Hz, consistently with NMR machine accuracy.
2.2 Synthesis
1H NMR (300 MHz, DMSO-d6) δ 0.8 (t, 3H, CH3), 1.25–1.5 (m, 6H), 1.8 (m, 2H), 3.5 (q, 2H), 6.1 (d, J = 8.7 MHz, 2H), 6.3 (s, NH), 8.5 (d, J = 8.7 MHz, 2H).
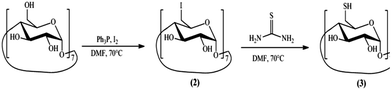
The symmetric derivatization of the β-CD is made through the synthesis of the intermediate per-6-iodo-β-cyclodextrin (2),25 by reaction with iodine and triphenylphosphine in DMF at 70 °C. The latter, in the presence of thiourea at 80 °C, originates the corresponding isothiouronium salt, which, following basic hydrolysis, forms the final per-6-deoxy-6-thio-β-cyclodextrin (3).26
1H NMR (300 MHz, DMSO-d6) δ 3.28 (t, J = 9 Hz, 7H), 3.34–3.48 (m, 14H), 3.54–3.68 (m, 14H), 3.80 (d, J = 9 Hz, 7H), 4.99 (d, J = 3 Hz, 7H), 5.94 (d, J = 2 Hz, 7H), 6.05 (d, J = 6.5 Hz, 7H).
1H NMR (300 MHz, DMSO-d6): δ 2.1 (t, J = 6 Hz, 8H, SH), 2.7 (m, 7H, H-6a), 3.2 (m, 7H, H-6b), 3.4 (m, 14H, H-2, H-4), 3.6–3.7 (m, 14H, H-3, H-5), 4.9 (d, J = 2.8 Hz, 7H, H-1), 5.8 (s, 7H, 3-OH), 5.9 (d, J = 6.7 Hz, 7H, 2-OH).
The so-obtained solid was dried in a pump and re-dissolved in DMF (50 mg in 2 mL, approximately 0.02 M). Molecule (1) (5 mg, 0.02 mmol) was then added and the solution was stirred for one day at room temperature. The reaction mixture was evaporated to dryness under reduced pressure, and the residue (QD/CD(3)/NBD(1)) washed with THF to remove non-included molecules.
2.3 Preparation of films of nano-hybrids in PVA matrix
Solutions of QD/CD(3)/NBD(1) and polyvinylalcool (PVA) in water were prepared at concentrations of 90 and 10 wt%, respectively. Each solution was then sonicated for 10 min, in order to obtain a uniform and stable dispersion of nano-hybrids in PVA. Films were prepared by casting 200 μL of the as-prepared solutions on quartz substrates.2.4 Morphological and optical characterization
AFM investigations were performed using a NT-MDT NTEGRA apparatus in tapping mode under ambient conditions with cantilevers with a resonant frequency of 80–200 kHz. HRTEM measurements performed with a ZEISS HRTEM LIBRA200.Optical absorption measurements were performed using a Perkin-Elmer Lambda-9 spectrometer. NBD molar extinction coefficient of 2.2 × 104 M−1 cm−1 at 455 nm has been measured in THF solution, while for QDs a value of 1.2 × 106 M−1 cm−1 at 500 nm in hexane is obtained.
Photoluminescence (PL) spectra were recorded by using a 270M SPEX spectrometer equipped with a N2 cooled SPEX Jobin Yvon CCD (charge-coupled device) detector, and by exciting with a Xenon lamp connected to a Jobin Yvon Gemini monochromator for the wavelength selection. The spectra were corrected for the instrument response.
The PL quantum yields (QY) on solid-state materials were obtained using a homemade integration sphere, following the procedure reported elsewhere,27 and a monochromated Xenon lamp. Photoluminescence excitation profiles (PLE) of the different systems were obtained by integrating the emission of the molecule while sweeping the excitation wavelengths. The integrations are then corrected with respect to the change in intensity of the lamp at different wavelengths by using a Rhodamine B solution as reference.
2.5 Computational methods
As an initial guess for the TD-DFT calculations of the CD/NBD(1) complex we used the molecular geometry of the NBD molecule (optimized at the CAM-B3LYP/6-31G** level) and the structure of β-CD derived from the X-ray diffraction pattern.28 The NBD(1) dye has been placed inside the β-CD cavity, with a distance between the NO2 group of the dye and one OH group of the β-CD shorter than 3 Å. At first a coarse optimization at MP3 level has been carried out, followed by a more accurate CAM-B3LYP/6-31G** optimization with the Gaussian 09 package.29 Optimizations have been carried out without geometrical constrains and with standard convergence criteria.A few other relative configurations have been tested all of which yielded less stable structures. In particular, by pulling out, by a sizeable amount, the head of the NBD(1) molecule and re-optimizing the geometry of the complex, we always end up with a stable geometry in which the NBD(1) molecule is inside the cavity. We can thus conclude that, provided the molecule enters the cavity a stable situation is found with the head forming hydrogen bonds with the β-CD OH groups and this causes a red shift of the excitation to the first excited state.
3. Results and discussion
The NBD–NH(CH2)5CH3 compound, hereafter NBD(1), has been synthesized by adding hexylamine to the starting NBD–Cl complex and exploiting the nucleophilic substitution of NBD–Cl halogens with primary amines (Scheme 2). This class of compounds are well-known for showing different fluorescence properties depending on the chemical groups used to functionalize the NBD skeleton.30,31 The successful inclusion of the as-synthesized NBD(1) derivative in the CD cavity has been demonstrated through PL measurements in the solid state and quantum-chemical calculations. As shown in Table 1, the fluorescence peak position of NBD(1) blue-shifts of about 60 nm and the PL QY increases from 0.7% to 26% when the molecule is included in the CD cavity (see also Fig. S1†). The low emission of NBD in the solid state is consistent with its environment sensitivity and with aggregation quenching phenomena that reduce the fluorescence properties of most organic dyes in the solid state.32 The emissive properties of the dyes can be recovered, in the solid state, by their insertion in a proper host. The organic host provides a protective environment and suppresses intermolecular interactions among the guests, so that their molecular fluorescence (typically the emission properties in diluted solutions) is restored.33 The PL properties of CD/NBD(1) films are comparable with those of NBD(1) solutions (Table S1†).The host–guest structure obtained with the inclusion of the organic dye into β-cyclodextrin is analysed by quantum-chemical calculations. A previous quantum mechanical study on the solvatochromic behavior of NBD(1) has shown that specific intermolecular interactions can affect the optical behavior of NBD(1).34 In particular, it was shown that the formation of H-bonds between NBD(1) and solvent molecules, leads to a decrease of the energy of the first dipole allowed excited state. TD-CAM-B3LYP/6-31G** calculations on the complex CD/NBD(1) indicate that the formation of a hydrogen bond between the head of the dye and one of the OH group of the β-CD leads to a decrease of the excitation energy. In particular, in the case of a complex in which the NO2 group of the dye forms a hydrogen bond with the OH of the β-CD (see Fig. 1), it is found that the excitation energy decreases by about 0.2 eV (S0–S1 transition 3.58 eV for the isolated molecule and 3.39 eV for the inclusion complex). This value correlates well with the observed decrease in the absorption maximum of the NBD(1) dye in solution with ethyl acetate (2.70 eV) with respect to the NBD(1) dye included in β-CD (2.56 eV). This complex configuration has been chosen because it is the most stable among those considered within this work. A complete mapping of the configuration space should have been undertaken, but, a complete study on the configurational space of the CD/NBD(1) inclusion complex is highly computationally demanding and it is out of the scope of the present investigation. The present calculations, however, indicate that the experimental optical spectra are consistent with the formation of the CD/NBD(1) inclusion complex.
In order to link the CD host to the QD surface, perthiolated β-cyclodextrin CD(3), where the primary hydroxyl groups of cyclodextrin have been substituted with thiols (see Scheme 3), has been prepared. The use of thiol groups to immobilize host molecules on the surface of different nanoparticles is a common strategy since thiols are known to have a great affinity for various nanoparticles surfaces.35,36 Thiols act as anchoring groups to replace the oleylamine ligands of CdSe/CdS core–shell QDs. The presence of the CD(3) host molecules on the QDs surface is confirmed by the drastic decrease of the PL QY of the QD/CD(3) nanocrystals (Table 1), while its absorption properties do not show relevant changes (Fig. S2†). In fact functionalization of the QD surface with thiols has been proven to quench the nanocrystals fluorescence through CT processes.12–14
TEM analysis of QDs (Fig. 2a) and cyclodextrin capped QDs (Fig. 2b) shows that particle dimensions fall between 8–10 nm. The dark-grey cloud where CdSe/CdS particles are embedded in Fig. 1b could be associated to cyclodestrine. In (c) the hystogram displaying the inter-particle distance distribution within the dark grey cloud shows that about one third of the particles are separated by distances higher than 10 nm, i.e., roughly the dimension of a CD capped particle. This suggests that about 30% of cyclodestrine surrounding the nanoparticles is not directly bonded to the QD. This excess of CD interacts with the neighbour shells thus forming a continuous cloud.
 | ||
| Fig. 2 HRTEM details of (a) QD and (b) QD/CD(3) particles. (c) Hystogram of interparticle distances from (b). | ||
The final hybrid system is then prepared by adding NBD(1) to the CD-capped QDs (Scheme 1). The molecule is included into the hydrophobic cavities of perthiolated β-cyclodextrines, both anchored to the QDs surface and free. The introduction of the neutral dye inside the hydrophobic walls of the cyclodextrin bound to the QD surface is able to switch the QD emission on (see Table 1). As shown later, an efficient RET process, competitive with the CT process, is introduced between the dye and the QD thus switching the emission on.
AFM morphology (Fig. 3) shows that particles deposited from solution in all the three cases (QD, QD/CD(3) and QD/CD(3)/NBD(1)) are mostly aggregated. However magnifications, see inlets in panel (a)–(c), of the different cases reveal a difference in shape: when covered by the CD shell or by the host–guest units, the particles become more spherical compared to the pristine case where particles appear more faceted. AFM images taken at different positions for each sample confirm the previous results.
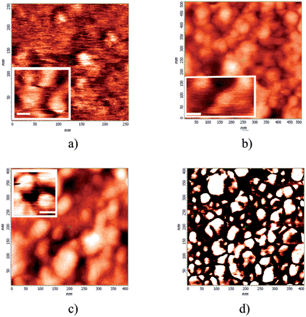 | ||
| Fig. 3 AFM morphology of (a) QD, (b) QD/CD(3), (c and d) QD/CD(3)/NBD(1) cast from solution. In (a–c) inlet, a magnification is reported where the line-bar corresponds to 15 nm. | ||
The optical properties of the hybrid host–guest system and its components are reported in Fig. 4. The absorption spectrum of the dye included in cyclodextrin is negligible at wavelengths longer than 530 nm, where the absorption spectrum of the hybrid system coincides with that of the QDs. On the other hand, below 530 nm, the spectrum of the complex shows the 470 nm band characteristic for the CD(3)/NBD(1) system. The green emission of the CD(3)/NBD(1) host–guest complex well overlaps with the broad absorption of the QDs, as requested in order to have efficient RET processes11,37 from the dye to the QDs. By exciting at 450 nm the hybrid composite, both the PL emissions characteristic of the dye included in CD(3) (560 nm) and of the QDs (620 nm) are observed. The presence of the host–guest emission band at 560 nm, in the hybrid film, is consistent with the presence of an excess of CD(3)/NBD(1) host–guest units. From the absorption spectrum of the hybrid film and the molar extinction coefficients of NBD(1) and QD, an experimental molar ratio of about 140 is obtained between NBD(1) and QDs while the molar ratio expected by assuming a complete coverage of the QD surface with host–guest units is about 50 (see ESI†). This observation is consistent with the HRTEM analysis (see Fig. 2b) showing that about 30% of free cyclodextrines forms a cloud embedding the nanoparticles. The distance among the host–guest units formed by these free CD(3) and the nanohybrids is larger than the Foerster radius37 (6.7 nm is obtained from the spectroscopic properties of the host–guest and QDs, see ESI†) and does not participate to the RET process. As a consequence an excess of host–guest units, whose distance is larger than the Foerster radius, are responsible of the 560 nm emission in the hybrid film.
 | ||
| Fig. 4 Absorption and PL spectra of cast films of QD/CD(3)/NBD(1) complex (black solid lines), CD(3)/NBD(1) host–guest (green dashed lines), CdSe/CdS QD (red dotted lines). PL are excited at 450 nm. | ||
Upon dispersion of the hybrids into the water soluble polymer polyvinylalcool (PVA), flat polymeric films are easily prepared embedding the nano-hybrids (see ESI†). The optical properties of the films are unaltered with respect to those of the hybrid powders,‡ thus opening to a variety of photonic and optoelectronic applications.
In order to evidence the harvesting properties of the organic host–guest on the QD emission, we have performed PL excitation profiles (PLE) of the hybrid complex measured at 655 nm, where only the contribution of the QD emission is present. In Fig. 5 we report the PLE spectra of the nano-hybrid dispersed in PVA films compared to the PLE of its two components CD(3)/NBD(1) and QD. The strict correspondence of the PLE spectrum of the QD emission in the complex with that of the CD(3)/NBD(1) host–guest demonstrates the presence of an efficient RET process from the dye to the QD in the complex. Interestingly, besides the widening of the emission spectrum (see Fig. 4) induced by the simultaneous emission of the host–guest units, the overall red-shift of the PLE profile of the hybrid film, with respect to the single QDs (see Fig. 5), allows to shift the excitation from 400 nm to 480 nm, better matching solar spectral properties for photovoltaic applications and reducing the use of harmful UV irradiation to produce the QD emission.38
4. Conclusions
We have reported a hybrid host–guest system based on a nitrobenzoxadiazole derivative dye and CdSe/CdS QDs covered with perthiolated β-cyclodextrin. CdSe/CdS QDs function as nanoscaffolds for the immobilization of cyclodextrin, in turn used as the host compound for the inclusion of a donor chromophore. In this hybrid system resonant energy transfer from the organic dye hosted in the cyclodextrin to the QD has been demonstrated, showing, for the first time, that CdSe/CdS QDs can be used as energy acceptors in a nanohybrid system. Further work on cyclodextrines derivatized with different functional groups is under investigation in order to enhance PL efficiency.Acknowledgements
This work was supported by Fondazione Cariplo Project 2010-0528 SOLCO.Notes and references
- (a) B. L. Cushing, V. L. Kolesnichenko and C. J. O'Connor, Chem. Rev., 2004, 104, 3893 CrossRef CAS PubMed; (b) A. M. Smith and S. Nie, Acc. Chem. Res., 2010, 43, 190–200 CrossRef CAS PubMed; (c) Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664–670 CrossRef CAS PubMed.
- (a) J. Kwon Oh, J. Mater. Chem., 2010, 20, 8433–8445 RSC; (b) M. Saba, S. Minniberger, F. Quochi, J. Roither, M. Marceddu, A. Gocalinska, M. V. Kovalenko, D. V. Talapin, W. Heiss, A. Mura and G. Bongiovanni, Adv. Mater., 2009, 21, 1–5 CrossRef.
- D. Dorokhin, S.-H. Hsu, N. Tomczak, D. N. Reinhoudt, J. Huskens, A. H. Velders and G. J. Vancso, ACS Nano, 2010, 4, 137–142 CrossRef CAS PubMed.
- J. Liu, G. Chen, M. Guo and M. Jiang, Macromolecules, 2010, 43, 8086–8093 CrossRef CAS.
- (a) K. A. Connors, Chem. Rev., 1997, 97, 1325 CrossRef CAS PubMed; (b) J. Szejtli, Chem. Rev., 1998, 98, 1743 CrossRef CAS PubMed; (c) F. Hapiot, S. Tilloy and E. Monflier, Chem. Rev., 2006, 106, 767 CrossRef CAS PubMed.
- (a) F. Cacialli, J. S. Wilson, J. J. Michels, C. Daniel, C. Silva, R. H. Friend, N. Severin, P. Samorì, J. P. Rabe, M. J. O'Connell, P. N. Taylor and H. L. Anderson, Nat. Mater., 2002, 1, 160–164 CrossRef CAS PubMed; (b) G. Fleury, C. Brochon, G. Schlatter, G. Bonnet, A. Lapp and G. Hadziioannou, Soft Matter, 2005, 1, 378–385 RSC.
- J. Aguilera-Sigalat, J. M. Casas-Solvas, M. C. Morant-Minana, A. Vargas-Berenguel, R. E. Galian and J. Pérez-Prieto, Chem. Commun., 2012, 48, 2573 RSC.
- (a) R. Freeman, T. Finder, L. Bahshi and I. Willner, Nano Lett., 2009, 9, 2073 CrossRef CAS PubMed; (b) D. Zhou, M. Lin, X. Liu, J. Li, Z. Chen, D. Yao, H. Sun, H. Zhang and B. Yang, ACS Nano, 2013, 7, 2273–2283 CrossRef CAS PubMed.
- (a) P. T. K. Chin, R. A. M. Hikmet, S. C. J. Meskers and R. A. J. Janssen, Adv. Funct. Mater., 2007, 17, 3829–3835 CrossRef CAS; (b) M. Achermann, S. Jeong, L. Balet, G. A. Montano and J. A. Hollingsworth, ACS Nano, 2011, 3, 1761–1768 CrossRef PubMed.
- A. R. Clapp, I. L. Mendintz and H. Mattoussi, ChemPhysChem, 2006, 7, 47–57 CrossRef CAS PubMed.
- (a) S. Halivni, A. Sitt, I. Hadar and U. Banin, ACS Nano, 2012, 6, 2758–2765 CrossRef CAS PubMed; (b) A. E. Albers, E. M. Chan, P. M. McBride, C. M. Ajo-Franklin, B. E. Cohen and B. A. Helms, J. Am. Chem. Soc., 2012, 134, 9565–9568 CrossRef CAS PubMed.
- (a) K. Palaniappan, S. A. Hackney and J. Liu, Chem. Commun., 2004, 270 Search PubMed; (b) R. C. Mulrooney, N. Singh, N. Kaur and J. F. Callan, Chem. Commun., 2009, 686–688 RSC.
- (a) J. Aguilera-Sigalat, V. F. Pais, A. Doménech-Carbó, U. Pischel, R. E. Galian and J. Pérez-Prieto, J. Phys. Chem. C, 2013, 117, 7365–7375 CrossRef CAS; (b) M. Saba, M. Aresti, F. Quochi, M. Marceddu, M. A. Loi, J. Huang, D. V. Talapin, A. Mura and G. Bongiovanni, ACS Nano, 2013, 7, 229–238 CrossRef CAS PubMed.
- I.-S. Liu, H.-H. Lo, C.-T. Chien, Y.-Y. Lin, C.-W. Chen, Y.-F. Chen, W.-F. Su and S.-C. Liou, J. Mater. Chem., 2008, 18, 675–682 RSC.
- F. M. Raymo and I. Yildiz, Phys. Chem. Chem. Phys., 2007, 9, 2036–2043 RSC.
- P. V. Kamat, J. Phys. Chem. C, 2008, 112, 18737–18753 CAS.
- A. R. Clapp, I. L. Medintz, H. T. Uyeda, B. R. Fisher, E. R. Goldman, M. G. Bawendi and H. Mattoussi, J. Am. Chem. Soc., 2005, 127, 18212 CrossRef CAS PubMed.
- E. Alphandery, L. M. Walsh, Y. Rakovich, A. L. Bradley, J. F. Donegan and N. Gaponik, Chem. Phys. Lett., 2004, 388, 100 CrossRef CAS PubMed.
- M. Hardzei, M. Artemeyev, M. Molinari, M. Troyon, A. Sukhanova and I. Nabiev, ChemPhysChem, 2012, 13, 330 CrossRef CAS PubMed.
- M. Artemeyev, E. Ustinovich and I. Nabiev, J. Am. Chem. Soc., 2009, 131, 8061 CrossRef PubMed.
- K. D. Wegner, P. T. Lanh, T. Jennings, E. Oh, V. Jain, S. M. Fairclough, J. M. Smith, E. Giovanelli, N. Lequeux, T. Pons and N. Hildebrandt, ACS Appl. Mater. Interfaces, 2013, 5, 2881–2892 CAS.
- (a) H. Xu, X. Huang, W. Zhang, G. Chen, W. Zhu and X. Zhong, ChemPhysChem, 2010, 11, 3167 CrossRef CAS PubMed; (b) M. Anni, L. Manna, R. Cingolani, D. Valerini, A. Creti and M. Lomascolo, Appl. Phys. Lett., 2004, 85, 4169–4171 CrossRef CAS PubMed.
- H. Gu, L. Bi, Y. Fu, N. Wang, S. Liu and Z. Tang, Chem. Sci., 2013, 4, 4371 RSC.
- (a) B. N. Pal, Y. Ghosh, S. Brovelli, R. Laocharoensuk, V. I. Klimov, J. A. Hollingsworth and H. Htoon, Nano Lett., 2012, 12, 331–336 CrossRef CAS PubMed; (b) F. Meinardi, A. Colombo, K. A. Velizhanin, R. Simonutti, M. Lorenzon, L. Beverina, R. Viswanatha, V. I. Klimov and S. Brovelli, Nat. Photonics, 2014, 8, 392–399 CrossRef CAS.
- P. R. Ashton, R. Königer and J. F. Stoddart, J. Org. Chem., 1996, 61, 903 CrossRef CAS.
- A. Benkhaled, H. Cheradame, O. Fichet, D. Teyssie, W. Buchmann and P. Guégan, Carbohydr. Polym., 2008, 73, 482 CrossRef CAS PubMed.
- J. Moreau, U. Giovanella, J.-P. Bombenger, W. Porzio, V. Vohra, L. Spadacini, G. Di Silvestro, L. Barba, G. Arrighetti, S. Destri, M. Pasini, M. Saba, F. Quochi, A. Mura, G. Bongiovanni, M. Fiorini, M. Uslenghi and C. Botta, ChemPhysChem, 2009, 10, 647 CrossRef CAS PubMed.
- R. W. Seidel and B. B. Koleva, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, o3162–o3163 CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- (a) S. Uchiyama, T. Santa, T. Fukushima, H. Homma and K. Imai, J. Chem. Soc., Perkin Trans. 2, 1998, 2165 RSC; (b) S. Uchiyama, T. Santa and K. Imai, J. Chem. Soc., Perkin Trans. 2, 1999, 2525 RSC.
- S. Uchiyama, K. Kimura, C. Gota, K. Okabe, K. Kawamoto, N. Inada, T. Yoshihara and S. Tobita, Chem.–Eur. J., 2012, 18, 9552–9563 CrossRef CAS PubMed.
- (a) R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed; (b) J. Gierschner, J. Cornil and H.-J. Egelhaaf, Adv. Mater., 2007, 19, 173–191 CrossRef CAS.
- (a) C. Botta, G. Patrinoiu, P. Picouet, S. Yunus, J. E. Communal, F. Cordella, F. Quochi, A. Mura, G. Bongiovanni, M. Pasini, S. Destri and G. Di Silvestro, Adv. Mater., 2004, 16, 1716–1721 CrossRef CAS; (b) C. Botta, P. Betti and M. Pasini, J. Mater. Chem. A, 2013, 1, 510–514 RSC; (c) P. Wang, X. Yan and F. Huang, Chem. Commun., 2014, 50, 5017–5019 RSC.
- C. Quarti, F. Villafiorita-Monteoleone, C. Botta, V. Daita, D. Perdicchia, P. Del Buttero and M. Del Zoppo, Chem. Phys. Lett. Search PubMed , submitted.
- J. Liu, W. Ong and A. E. Kaifer, Langmuir, 2002, 18, 5981 CrossRef CAS.
- M.-X. Zhao, H. Su, Z.-W. Mao and L.-N. Ji, J. Lumin., 2012, 132, 16 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic Plenum Publishers, New York, 1999 Search PubMed.
- K. Palaniappan, C. Xue, G. Arumugam, S. A. Hackney and J. Liu, Chem. Mater., 2006, 18, 1275–1280 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Optical characterization. See DOI: 10.1039/c4ra03930k |
| ‡ PL spectra in PVA blends are blue shifted of 10 nm, with respect to the hybrid powders. |
| This journal is © The Royal Society of Chemistry 2014 |