Tailored recovery of carbons from waste tires for enhanced performance as anodes in lithium-ion batteries†
Abstract
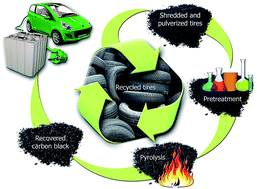
Morphologically tailored pyrolysis-recovered carbon black is utilized in lithium-ion battery anodes with improved capacity as a potential solution for adding value to waste tire-rubber-derived materials. Micronized tire rubber was digested in a hot oleum bath to yield a sulfonated rubber slurry that was then filtered, washed, and compressed into a solid cake. Carbon was recovered from the modified rubber cake by pyrolysis in a nitrogen atmosphere. The chemical pretreatment of rubber produced a carbon monolith with higher yield than that from the control (a fluffy tire-rubber-derived carbon black). The carbon monolith showed a very small volume fraction of pores of widths 3–5 nm, prominent nanoporosity (pore width < 2 nm), reduced specific surface area, and an ordered assembly of graphitic domains. Electrochemical studies revealed that the recovered-carbon-based anode had a higher reversible capacity than that of graphite. Anodes made with a sulfonated tire-rubber-derived carbon and a control tire-rubber-derived carbon exhibited an initial coulombic efficiency of 71% and 45%, respectively. The reversible capacity of the cell with the sulfonated tire rubber-derived carbon as the anode was 390 mA h g−1 after 100 cycles, with nearly 100% coulombic efficiency. Our success in producing a higher performance carbon material from waste tire rubber for potential use in energy storage applications adds a new avenue to tire rubber recycling.

 Please wait while we load your content...
Please wait while we load your content...