Catalyst-free cascade reaction of heterocyclic ketene aminals with N-substituted maleimide to synthesise bicyclic pyrrolidinone derivatives†
Jin Liu,
Hai-Rui Zhang,
Xin-Rong Lin,
Sheng-Jiao Yan* and
Jun Lin*
Key Laboratory of Medicinal Chemistry for Natural Resource (Yunnan University), Ministry Education, School of Chemical Science and Technology, Yunnan University, Kunming, 650091, P. R. China. E-mail: yansj@ynu.edu.cn; linjun@ynu.edu.cn; Fax: +86 871 65033215
First published on 11th June 2014
Abstract
An efficient synthesis of highly substituted bicyclic pyrrolidinone derivatives via a cascade reaction of heterocyclic ketene aminals (HKAs) and N-substituted maleimide in an environmentally friendly medium under catalyst-free conditions is described. This protocol uses group-assisted purification (GAP) chemistry in which purification via chromatography and recrystallization can be avoided, and the pure products were obtained simply by washing the crude products with 95% ethanol. The library of bicyclic pyrrolidinone derivatives has been constructed with moderate to excellent yields.
Introduction
The 2-pyrrolidinone nucleus is one of the most represented structural motifs in naturally-occurring compounds and serves as a key synthetic intermediate or as a pharmacophore in drug discovery processes.1 Among 2-pyrrolidinone derivatives, bicyclic pyrrolidinone derivatives as natural products have been widely researched, such as pyrrolams A–D (Fig. 1), which were isolated by Zeeck and co-workers in 1990 from the bacterial strain Streptomyces olivaceus. Bioassays revealed modest biological activity.2a Bicyclic pyrrolidinone derivatives have been used as anti-biotic agents (Carbapenem, Fig. 1),2b human NK1 antagonists (Fig. 1),3 proteasome inhibitors,4 anti-seizure agents,5 and transcription-factor inhibitors.6Owing to the synthetic utilization and biological importance of the bicyclic pyrrolidinones, several methods for the construction of these kinds of compounds with diverse structural features and substitution patterns have been developed over the past decade. Synthetic approaches include radical or electrophilic cyclisations of unsaturated amides,7 direct reaction of imines with cyclic anhydrides,8 Au-catalysed cyclisations,9 carbenoid C–H insertions cyclisations10 and ring expansions.11 However, many of these methods involve the use of expensive or toxic transition metals as catalysts (Rh, Au), extended reaction times, high temperatures, and also require tedious work-up procedures. Consequently, an efficient, concise and environmentally friendly approach for producing this class of bicyclic pyrrolidinones that tolerate a wide variety of functional groups is highly desirable.
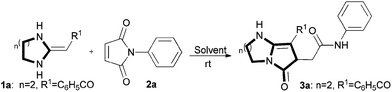
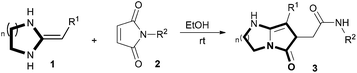
In the past several years, our groups have demonstrated that heterocyclic ketene aminals (HKAs) are an emerging, more reactive class of functionalized synthons12 through which a variety of biologically active heterocyclic13 and fused heterocyclic compounds can be obtained, using easier and more efficient methodologies.14,15 One concern of our group is synthesis of diverse compound libraries using green chemistry techniques under mild reaction conditions. The green synthesis can usually avoid tedious workup and purifications. In this paper we report a method for a concise synthesis of bicyclic pyrrolidinone derivatives. The pure products were obtained by relying on group-assisted purification (GAP) chemistry16 to avoid traditional purification methods of column chromatography or recrystallization (Scheme 1).
Results and discussion
Initially, HKA 1a was reacted with the easily accessible material N-phenylmaleimide 2a in acetone in the presence of Et3N at room temperature. After 40 min, a light yellow solid was obtained with a 70% yield after separation by filtration (Table 1, entry 1). To establish the optimal reaction conditions, acid catalysts HOAc and a catalyst-free condition were involved (Table 1, entry 2–3). We found that the reactions could proceed in catalyst-free conditions, while the catalysts Et3N and AcOH didn't obviously promote the reactions (Table 1, entries 1–2 vs. 3). Subsequently, we screened other solvents by still using a catalyst-free condition at room temperature (Table 1, entries 4–10). From the various entries, we believe that ethanol is a green solvent, and that room temperature is beneficial for reducing energy consumption and for convenient operation. Therefore, we propose that the best reaction conditions for the synthesis of fused bicyclic pyrrolidinones are EtOH as a solvent, a catalyst-free condition, at ambient temperature for 20 min to obtain products with an 84% isolated yield (Table 1, entry 6).| Entry | Solvent | Catalyst | t (°C) | Time/min | Yieldb (%) |
|---|---|---|---|---|---|
| a Reagents and conditions: HKA 1a (0.50 mmol), N-phenylmaleimide 2a (0.55 mmol), solvent (15.0 mL).b Isolated yield based on HKA 1a. | |||||
| 1 | Acetone | Et3N | r.t. | 40 | 70 |
| 2 | Acetone | AcOH | r.t. | 40 | 67 |
| 3 | Acetone | — | r.t. | 40 | 72 |
| 4 | CH3CN | — | r.t. | 120 | 25 |
| 5 | AcOEt | — | r.t. | 120 | 20 |
| 6 | EtOH | — | r.t. | 20 | 84 |
| 7 | EtOH–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | r.t. | 30 | 77 |
| 8 | MeOH | — | r.t. | 10 | 35 |
| 9 | CH2Cl2 | — | r.t. | 60 | 65 |
| 10 | Dioxane | — | r.t. | 120 | 34 |
Based on the optimisation conditions, the scope and limitations of this protocol have been examined, and a number of six-membered ring HKAs 1b–f were used as substrates to react with N-phenylmaleimide 2a and b. The results demonstrated that the six-membered HKAs, with different substituents, were all good substrates for the cascade reaction at ambient temperature (Table 2, entries 1–12). The substituents of the HKAs 1 also had a slight influence on the reactivity and product yield. After that, N-(4-substituted phenyl) maleimides 2c and d, N-ethylmaleimide 2e and maleimide 2f were reacted with six-membered ring HKAs under the same conditions. In the long run, the reaction can provide the target compounds in good yields but the reaction times need to be extended (Table 2, entries 13–21).
| Entry | n | R1 | R2 | 3 | Time/min | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reagents and conditions: HKA 1 (0.50 mmol), N-phenylmaleimide 2 (0.55 mmol), solvent (15.0 mL).b Isolated yield based on HKA 1.c Isolated yields after column chromatography. | ||||||
| 1 | 2 | C6H5CO (1a) | C6H5 (2a) | 3a | 20 | 84 |
| 2 | 2 | 4-FC6H4CO (1b) | C6H5 (2a) | 3b | 20 | 70 |
| 3 | 2 | 4-ClC6H4CO (1c) | C6H5 (2a) | 3c | 20 | 85 |
| 4 | 2 | 2-ClC6H4CO (1d) | C6H5 (2a) | 3d | 20 | 83 |
| 5 | 2 | 4-MeC6H4CO (1e) | C6H5 (2a) | 3e | 20 | 92 |
| 6 | 2 | 4-MeOC6H4CO (1f) | C6H5 (2a) | 3f | 20 | 73 |
| 7 | 2 | C6H5CO (1a) | Benzyl (2b) | 3g | 20 | 95 |
| 8 | 2 | 4-FC6H4CO (1b) | Benzyl (2b) | 3h | 20 | 80 |
| 9 | 2 | 4-ClC6H4CO (1c) | Benzyl (2b) | 3i | 20 | 88 |
| 10 | 2 | 2-ClC6H4CO (1d) | Benzyl (2b) | 3j | 20 | 92 |
| 11 | 2 | 4-MeC6H4CO (1e) | Benzyl (2b) | 3k | 20 | 90 |
| 12 | 2 | 4-MeOC6H4CO (1f) | Benzyl (2b) | 3l | 20 | 94 |
| 13 | 2 | C6H5CO (1a) | 4-FC6H4 (2c) | 3m | 60 | 72 |
| 14 | 2 | 4-FC6H4CO (1b) | 4-FC6H5 (2c) | 3n | 60 | 85 |
| 15 | 2 | 4-MeOC6H4CO (1f) | 4-FC6H4 (2c) | 3o | 60 | 93 |
| 16 | 2 | C6H5CO (1a) | 4-MeOC6H4 (2d) | 3p | 120 | 94 |
| 17 | 2 | 4-FC6H4CO (1b) | 4-MeOC6H4 (2d) | 3q | 60 | 84 |
| 18 | 2 | 4-MeOC6H4CO (1f) | 4-MeOC6H4 (2d) | 3r | 60 | 79 |
| 19 | 2 | C6H5CO (1a) | Ethyl (2e) | 3s | 60 | 96 |
| 20 | 2 | 4-FC6H4CO (1b) | Ethyl (2e) | 3t | 60 | 85 |
| 21 | 2 | C6H5CO (1a) | H (2f) | 3u | 60 | 79 |
| 22 | 3 | 4-FC6H4CO (1b) | C6H5 (2a) | 3v | 90 | 78 |
| 23 | 3 | 4-ClC6H4CO (1c) | C6H5 (2a) | 3w | 90 | 77 |
| 24 | 3 | 4-MeC6H4CO (1e) | C6H5 (2a) | 3x | 90 | 82 |
| 25 | 3 | 4-FC6H4CO (1b) | Benzyl (2b) | 3y | 90 | 90 |
| 26 | 3 | 4-MeC6H4CO (1e) | Benzyl (2b) | 3z | 90 | 92 |
| 27 | 1 | C6H5CO (1a) | C6H5 (2a) | 3a′ | 60 | 28c |
| 28 | 1 | 4-MeOC6H4CO (1f) | C6H5 (2a) | 3b′ | 60 | 32c |
| 29 | 1 | C6H5CO (1a) | 4-FC6H4 (2c) | 3c′ | 60 | 71 |
| 30 | 1 | C6H5CO (1a) | 4-MeOC6H4 (2d) | 3d′ | 60 | 76 |
| 31 | 1 | 4-MeOC6H4CO (1f) | Benzyl (2d) | 3e′ | 60 | 20c |
| 32 | 1 | C6H5CO (1a) | Ethyl (2e) | 3f′ | 30 | 77 |
| 33 | 1 | 4-ClC6H4CO (1c) | Ethyl (2e) | 3g′ | 30 | 78 |
| 34 | 1 | 4-MeOC6H4CO (1f) | Ethyl (2e) | 3h′ | 30 | 80 |
Inspired by the obtained results, ring size was also investigated in our work. Thus, the seven-membered HKAs were reacted with N-phenylmaleimide 2a and N-benzylmaleimide 2b. The reactions proceeded smoothly under the same conditions and we attained a final product with good yields, albeit with prolonged reaction times (Table 2, entries 22–26). However, when the five-member HKAs were applied, the reaction produced a complicated mixture of products, and we obtained the final product by column chromatography with ethyl acetate as an eluent to afford bicyclic pyrrolidinones with low yields (Table 2, entries 27–28). More significantly, the five-member HKAs were reacted with N-(4-substituted phenyl) maleimides 2c and d, and N-ethylmaleimide 2e under the same conditions. The pure products were obtained by relying on GAP chemistry with moderate yields (Table 2, entries 32–34).
The 1H, 13C NMR spectra, IR spectra and high resolution mass spectra data have confirmed the structure of the target compound 3. In order to specifically test the structure, 3t was characterised by X-ray crystallography as a representative compound, as shown in Fig. 2.
A proposed mechanism for the synthesis of bicyclic pyrrolidinone derivatives 3 is shown in Scheme 2. Firstly, the α-C of HKAs 1 adds to the double bond of N-phenylmaleimide 2 and affords 4 via a Michael addition reaction. Secondly, the intermediate 4 is followed by imine–enamine tautomerization17 to give compound 5. Finally, the NH group of compound 5 attacks the amide carbonyl group via an intramolecular cyclisation reaction accompanied with ring-opening reaction to bicyclic pyrrolidinone 3.
Conclusions
In summary, a concise method for the synthesis of a series of bicyclic pyrrolidinones via HKAs and N-substituted maleimide at room temperature has been developed. The reaction showed that the synthetic route allowed the construction of fused bicyclic-pyrrolidinone derivatives with a wide range of substituents as important building blocks. Features of this strategy include some important aspects like convenient operation, short reaction times, green solvent, absence of catalysts and simple purification by washing the crude products with minimum amounts of common solvents, defined as GAP (group-assisted purification) chemistry. The library of bicyclic pyrrolidinones has been constructed with satisfactory yields.Experimental section
All compounds were fully characterized by spectroscopic data. The NMR spectra were recorded on a Bruker DRX500 (1H: 500 MHz, 13C: 125 MHz). Chemical shifts (δ) are expressed in ppm and J values are given in Hz. Deuterated DMSO-d6 was used as solvent. IR spectra were recorded on a FT-IR Thermo Nicolet Avatar 360 using a KBr pellet. The reactions were monitored by thin layer chromatography (TLC) using silica gel GF254. The melting points were determined on a XT-4A melting point apparatus and are uncorrected. HRMs were performed on an Agilent LC/Msd TOF instrument. All chemicals and solvents were used as received without further purification unless otherwise stated. Compounds 1 were prepared according to the literature.18 Materials 2 were synthesized according with the literature.19General procedure
HKA derivatives 1 (0.50 mmol), N-substituted maleimides 2 (0.55 mmol) and ethanol (15 mL) were placed into a 25 mL round-bottom flask and the mixture was stirred at room temperature for 20–120 min. Completion of the reaction was monitored by TLC. The reaction mixture was then filtered to obtain the pure crude product, which was further washed with 95% EtOH to give pure product 3 with a yield of 20–96%. The products were further identified by FTIR, NMR and HRMS.2-(8-Benzoyl-6-oxo-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyr-imidin-7-yl)-N-phenylacetamide (3a)
Light yellow solid: mp 191–193 °C; IR (KBr): 3424, 3244, 1740, 1632, 1526, 1434, 1158, 1082, 745 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.81 (br, 1H, NH), 9.54 (br, 1H, NH), 7.52–7.51 (m, 2H, ArH), 7.41–7.38 (m, 5H, ArH), 7.23–7.22 (m, 2H, ArH), 6.99–6.96 (m, 1H, ArH), 4.03–3.95 (m, 1H, CH), 3.59–3.55 (m, 2H, NCH2), 3.51–3.42 (m, 2H, CH2N), 2.54–2.23 (m, 2H, CH2CO), 1.95–1.89 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.8, 177.6, 168.4, 158.6, 142.3, 139.3, 129.6, 129.0, 128.5, 126.9, 123.3, 119.4, 88.5, 40.8, 38.7, 37.3, 36.9, 19.9; HRMS (TOF ES+): m/z calcd for C22H21N3NaO3 [(M + Na)+], 398.1475; found, 398.1472.2-(8-(4-Fluorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrrolo-[1,2-a]pyrimidin-7-yl)-N-phenylacetamide (3b)
Light yellow solid: mp 192–193 °C; IR (KBr): 3313, 2933, 1733, 1619, 1537, 1441, 1149, 853, 760 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.80 (br, 1H, NH), 9.53 (br, 1H, NH), 7.59–7.56 (m, 2H, ArH), 7.40–7.39 (m, 2H, ArH), 7.24–7.19 (m, 4H, ArH), 6.99–6.96 (m, 1H, ArH), 4.00–3.96 (m, 1H, CH), 3.58–3.55 (m, 2H, NCH2), 3.43–3.41 (m, 2H, CH2N), 2.57–2.27 (m, 2H, CH2CO), 1.94–1.92 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.5, 177.1, 168.3, 161.9, 158.8, 139.2, 138.7, 129.3, 128.9, 123.4, 119.4, 115.4 (d, J = 21.3 Hz), 88.4, 40.8, 38.7, 37.3, 37.0, 19.9; HRMS (TOF ES+): m/z calcd for C22H20FN3NaO3 [(M + Na)+], 416.1381; found, 416.1381.2-(8-(4-Chlorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrrolo-[1,2-a]pyrimidin-7-yl)-N-phenylacetamide (3c)
Light yellow solid: mp 211.5–212.5 °C; IR (KBr): 3369, 3228, 1735, 1635, 1525, 1368, 1269, 1160, 1086, 772, 715 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.81 (br, 1H, NH), 9.55 (br, 1H, NH), 7.55–7.53 (m, 2H, ArH), 7.43–7.38 (m, 4H, ArH), 7.24–7.21 (m, 2H, ArH), 6.99–6.96 (m, 1H, ArH), 3.96–3.96 (m, 1H, CH), 3.59–3.55 (m, 2H, NCH2), 3.43–3.42 (m, 2H, CH2N), 2.60–2.29 (m, 2H, CH2CO), 1.96–1.1.91 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.2, 177.1, 168.3, 159.0, 141.0, 139.2, 134.1, 128.9, 128.9, 128.6, 123.4, 119.4, 88.6, 40.7, 38.7, 37.4, 37.1, 19.9; HRMS (TOF ES+): m/z calcd for C22H20ClN3NaO3 [(M + Na)+], 432.1085; found, 432.1086.2-(8-(2-Chlorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrrolo-[1,2-a]pyrimidin-7-yl)-N-phenylacetamide (3d)
Light yellow solid: mp 190–190.6 °C; IR (KBr): 3364, 3225, 1737, 1635, 1525, 1160, 1086, 772 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.62 (br, 1H, NH), 9.52 (br, 1H, NH), 7.45–7.42 (m, 3H, ArH), 7.35–7.32 (m, 2H, ArH), 7.25–7.22 (m, 3H, ArH), 7.00–6.97 (m, 1H, ArH), 3.59–3.56 (m, 2H, CH2N), 3.55–3.53 (m, 1H, CH), 3.49–3.43 (m, 2H, NCH2), 2.44–2.03 (m, 2H, CH2CO), 1.96–1.91 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.7, 177.4, 168.6, 158.6, 141.8, 139.8, 130.5, 130.5, 130.4, 129.4, 128.8, 128.0, 123.8, 120.0, 89.6, 40.7, 39.3, 37.8, 37.3, 20.3; HRMS (TOF ES+): m/z calcd for C22H20ClN3NaO3 [(M + Na)+], 432.1085; found, 432.1086.2-(8-(4-Methylbenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrrolo-[1,2-a]pyrimidin-7-yl)-N-phenylacetamide (3e)
Light yellow solid: mp 215–218 °C; IR (KBr): 3256, 3120, 1738, 1683, 1631, 1525, 1439, 1160, 1094, 837, 759 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.81 (br, 1H, NH), 9.52 (br, 1H, NH), 7.44–7.40 (m, 4H, ArH), 7.24–7.22 (m, 2H, ArH), 7.21–7.18 (m, 2H, ArH), 6.99–6.96 (m, 1H, ArH), 4.03–3.99 (m, 1H, CH), 3.57–3.54 (m, 2H, CH2N), 3.42 (m, 2H, NCH2), 2.53–2.28 (m, 2H, CH2CO), 2.30 (s, 3H CH3), 2.00–1.87 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 183.3, 177.7, 168.9, 159.0, 139.8, 139.4, 129.8, 129.4, 127.6, 123.8, 124.6, 119.8, 88.9, 41.3, 40.0, 37.8, 37.6, 21.8, 20.5; HRMS (TOF ES+): m/z calcd for C23H24N3O3 [(M + H)+], 390.1812; found, 390.1816.2-(8-(4-Methoxybenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrro-lo[1,2-a]pyrimidin-7-yl)-N-phenylacetamide (3f)
Light yellow solid: mp 207–209 °C; IR (KBr): 3334, 3244, 1731, 1638, 1526, 1438, 1161, 1090, 760 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.79 (br, 1H, NH), 9.54 (br, 1H, NH), 7.51 (d, J = 8.5 Hz, 2H, ArH), 7.40 (d, J = 8.2 Hz, 2H, ArH), 7.23–7.20 (m, 2H, ArH), 6.99–6.96 (m, 1H, ArH), 6.91 (d, J = 8.6 Hz, 2H, ArH), 4.04–3.99 (m, 1H, CH), 3.76 (s, 3H, OCH3), 3.61–3.49 (m, 4H, NCH2CH2N), 2.57–2.33 (m, 2H, CH2CO), 1.96–1.89 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 187.5, 182.5, 175.1, 165.6, 163.8, 144.5, 139.8, 134.2, 134.2, 128.6, 124.6, 119.0, 95.5, 60.7, 46.2, 43.9, 42.6, 42.3, 25.2; HRMS (TOF ES+): m/z calcd for C23H24N3O4 [(M + H)+], 406.1761; found, 406.1757.2-(8-Benzoyl-6-oxo-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyr-imidin-7-yl)-N-benzylacetamide (3g)
Light yellow solid: mp 209–211.5 °C; IR (KBr): 3256, 3060, 2917, 1728, 1635, 1526, 1442, 1159, 907, 742 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.83 (br, 1H, NH), 7.92 (br, 1H, NH–Bn), 7.55–7.50 (m, 2H, ArH), 7.42–7.36 (m, 3H, ArH), 7.30–7.27 (m, 2H, ArH), 7.22–7.21 (m, 1H, ArH), 7.11–7.09 (m, 2H, ArH), 4.19–4.01 (m, 2H, CH2Ph), 3.93–3.91 (m, 1H, CH), 3.62–3.45 (m, 2H, CH2N), 3.40–3.39 (m, 2H, NCH2), 2.39–2.11 (m, 2H, CH2CO), 1.98–1.85 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 183.3, 177.7, 169.8, 159.2, 142.8, 140.25, 130.0, 129.0, 127.7, 127.5, 89.0, 42.6, 41.4, 39.6, 37.8, 36.4, 20.4; HRMS (TOF ES+): m/z calcd for C23H23N3NaO3 [(M + Na)+], 412.1632; found, 412.1635.N-Benzyl-2-(8-(4-fluorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahy-dropyrrolo[1,2-a]pyrimidin-7-yl)acetamide (3h)
Light yellow solid: mp 218–222 °C; IR (KBr): 3236, 3052, 1734, 1637, 1526, 1446, 1099, 845, 768 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.83 (br, 1H, NH), 7.94 (br, 1H, NH–Bn), 7.60–7.58 (m, 2H, ArH), 7.31–7.28 (m, 2H, ArH), 7.22–7.19 (m, 3H, ArH), 7.09–7.08 (m, 2H, ArH), 4.18–4.01 (m, 2H, CH2Ph), 3.93–3.91 (m, 1H, CH), 3.62–3.45 (m, 4H, CH2N), 2.44–2.15 (m, 2H, CH2CO), 2.01–1.81 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.4, 177.2, 169.2, 163.8, 158.9, 139.7, 138.8, 129.4, 128.5, 127.2, 126.9, 115.3 (d, J = 21.3 Hz), 88.4, 42.1, 40.9, 38.7, 37.3, 35.9, 19.9; HRMS (TOF ES+): m/z calcd for C23H22FN3NaO3 [(M + Na)+], 430.1537; found, 430.1537.N-Benzyl-2-(8-(4-chlorobenzoyl)-6-oxo-1,2,3,4,6,7-hexa-hydropyrrolo[1,2-a]pyrimidin-7-yl)acetamide (3i)
Light yellow solid: mp 232–234 °C; IR (KBr): 3366, 3227, 1736, 1635, 1525, 1086, 772 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.84 (br, 1H, NH), 7.95 (br, 1H, NH–Bn), 7.56–7.54 (m, 2H, ArH), 7.44–7.42 (m, 2H, ArH), 7.31–7.28 (m, 2H, ArH), 7.21–7.19 (m, 1H, ArH), 7.09–7.08 (m, 2H, ArH), 4.20–4.01 (m, 2H, CH2Ph), 3.91 (m, 1H, CH), 3.63–3.49 (m, 2H, CH2N), 3.40 (m, 2H, NCH2), 2.55–2.16 (m, 2H, CH2CO), 2.00–1.80 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.1, 177.1, 169.2, 159.1, 141.0, 139.7, 134.1, 129.0, 128.5, 128.5, 127.2, 126.9, 88.6, 42.1, 40.8, 38.7, 37.3, 35.9, 19.8; HRMS (TOF ES+): m/z calcd for C23H22ClN3NaO3 [(M + Na)+], 446.1242; found, 446.1240.N-Benzyl-2-(8-(2-chlorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahy-dropyrrolo[1,2-a]pyrimidin-7-yl)acetamide (3j)
Light yellow solid: mp 199–202 °C; IR (KBr): 3363, 3226, 1738, 1635, 1525, 1086, 772 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.51 (br, 1H, NH), 8.02 (br, 1H, NH–Bn), 7.46–7.42 (m, 1H, ArH), 7.36–7.30 (m, 4H, ArH), 7.23–7.18 (m, 4H, ArH), 4.18–7.07 (m, 2H, CH2Ph), 3.56–3.52 (m, 1H, CH), 3.52–3.46 (m, 4H, NCH2CH2N), 2.28–1.93 (m, 2H, CH2CO), 1.93–1.89 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.7, 177.5, 169.6, 158.6, 141.9, 140.3, 130.5, 130.4, 130.4, 129.0, 129.0, 127.9, 127.5, 89.7, 42.8, 40.5, 39.2, 37.7, 36.2, 20.3; HRMS (TOF ES+): m/z calcd for C23H22ClN3NaO3 [(M + Na)+], 446.1242; found, 446.1241.N-Benzyl-2-(8-(4-methylbenzoyl)-6-oxo-1,2,3,4,6,7-hexahy-dropyrrolo[1,2-a]pyrimidin-7-yl)acetamide (3k)
Light yellow solid: mp 179–181 °C; IR (KBr): 3559, 3420, 3227, 1724, 1633, 1521, 1450, 1099, 756 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.85 (br, 1H, NH), 7.94 (br, 1H, NH–Bn), 7.47–7.45 (d, J = 7.9 Hz, 2H, ArH), 7.30–7.27 (m, 2H, ArH), 7.21–7.18 (m, 3H, ArH), 7.11–7.10 (d, J = 7.6 Hz, 2H, ArH), 4.19–4.02 (m, 2H, CH2Ph), 3.95–3.94 (m, 1H, CH), 3.61–3.44 (m, 2H, CH2N), 3.47–3.45 (m, 2H, NCH2), 2.40–2.16 (m, 2H, CH2CO), 2.32 (s, 3H, CH3), 1.94–1.85 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.7, 177.2, 169.4, 158.0, 139.7, 139.5, 139.0, 129.0, 128.5, 127.2, 127.1, 126.9, 88.4, 42.1, 41.0, 38.6, 37.3, 35.9, 21.4, 19.9; HRMS (TOF ES+): m/z calcd for C24H25N3NaO3 [(M + Na)+], 426.1788; found, 426.1790.N-Benzyl-2-(8-(4-methoxybenzoyl)-6-oxo-1,2,3,4,6,7-hexa-hydropyrrolo[1,2-a]pyrimidin-7-yl)acetamide (3l)
Light yellow solid: mp 216–219 °C; IR (KBr): 3285, 3199, 2917, 1735, 1632, 1518, 1433, 1360, 1258, 1161, 1198, 1023, 849, 765, 613 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.83 (br, 1H, NH), 7.91 (br, 1H, NH–Bn), 7.54–7.52 (d, J = 8.6 Hz, 2H, ArH), 7.31–7.25 (m, 1H, ArH), 7.21–7.20 (m, 1H, ArH), 7.10–7.09 (m, 2H, ArH), 6.91 (d, J = 8.7 Hz, 2H, ArH), 4.18–4.02 (m, 2H, CH2Ph), 3.95 (m, 1H, CH), 3.78 (s, 3H, OCH3), 3.60–3.47 (m, 2H, CH2N), 3.39–3.38 (m, 2H, NCH2), 2.45–2.21 (m, 2H, CH2CO), 1.97–1.81 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.1, 177.2, 169.4, 160.4, 158.6, 139.7, 134.7, 128.9, 128.5, 127.2, 126.9, 113.6, 88.1, 55.5, 42.1, 41.1, 38.6, 37.3, 35.9, 20.0; HRMS (TOF ES+): m/z calcd for C24H25N3NaO4 [(M + Na)+], 442.1737; found, 442.1739.2-(8-Benzoyl-6-oxo-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyr-imidin-7-yl)-N-(4-fluorophenyl)acetamide (3m)
Light yellow solid: mp 190.5–191.5 °C; IR (KBr): 3264, 3068, 1728, 1631, 1519, 1088, 837 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.81 (br, 1H, NH), 9.61 (br, 1H, NH), 7.52–7.50 (m, 2H, ArH), 7.47–7.35 (m, 5H, ArH), 7.07–7.04 (m, 2H, ArH), 4.04–3.94 (m, 1H, CH), 3.64–3.48 (m, 2H, CH2N), 3.42–3.41 (m, 2H, NCH2), 2.54–2.21 (m, 2H, CH2CO), 1.97–1.89 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 183.3, 177.6, 168.8, 159.1, 157.7, 142.8, 136.2, 130.1, 129.0, 127.4, 121.5, 116.0 (d, J = 21.3 Hz), 88.9, 41.2, 39.2, 37.8, 37.3, 20.4; HRMS (TOF ES+): m/z calcd for C22H20FN3NaO3 [(M + Na)+], 416.1381; found, 416.1380.2-(8-(4-Fluorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrrolo-[1,2-a]pyrimidin-7-yl)-N-(4-fluorophenyl)acetamide (3n)
Light yellow solid: mp 129–132 °C; IR (KBr): 3267, 3076, 1733, 1636, 1515, 1440, 1267, 1224, 1158, 1082, 841, 772, 608, 510 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.80 (br, 1H, NH), 9.59 (br, 1H, NH), 7.59–7.56 (m, 2H, ArH), 7.47–7.37 (m, 2H, ArH), 7.20–7.17 (m, 2H, ArH), 7.08–7.05 (m, 2H, ArH), 3.99–3.97 (m, 1H, CH), 3.62–3.50 (m, 2H, CH2N), 3.43–3.42 (m, 2H, NCH2), 2.57–2.25 (m, 2H, CH2CO), 2.00–1.84 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.5, 177.1, 168.2, 162.9 (d, J = 245.0 Hz), 158.8, 158.2 (d, J = 238.8 Hz), 138.7, 135.6, 129.4, 121.1, 115.4 (d, J = 21.3 Hz), 115.3, 88.4, 40.8, 38.7, 37.3, 37.0, 19.9; HRMS (TOF ES+): m/z calcd for C22H19F2N3NaO3 [(M + Na)+], 434.1287; found, 3434.1287.N-(4-Fluorophenyl)-2-(8-(4-methoxybenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyrimidin-7-yl)acetamide (3o)
Light yellow solid: mp 182–184.5 °C; IR (KBr): 3264, 3211, 3076, 1733, 1633 1512, 1428, 1255, 1160, 1090, 1012, 845, 767, 600, 498 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.81 (br, 1H, NH), 9.60 (br, 1H, NH), 7.53 (d, J = 8.4 Hz, 2H, ArH), 7.45–7.42 (m, 2H, ArH), 7.09–7.05 (m, 2H, ArH), 6.93–6.91 (d, J = 8.4, 2H, ArH), 4.04–4.02 (m, 1H, CH), 3.77 (s, 3H, OCH3), 3.55–3.54 (m, 2H, CH2N), 3.43–3.42 (m, 2H, NCH2), 2.58–2.33 (m, 2H, CH2CO), 1.98–1.86 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.2, 177.2, 168.3, 160.4, 158.5, 135.7, 134.6, 128.8, 121.1, 115.5, 115.4, 113.7, 88.1, 55.4, 41.0, 38.6, 37.3, 37.0, 20.0; HRMS (TOF ES+): m/z calcd for C23H22FN3NaO4 [(M + Na)+], 446.1487; found, 446.1485.2-(8-Benzoyl-6-oxo-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyr-imidin-7-yl)-N-(4-methoxyphenyl)acetamide (3p)
Light yellow solid: mp 184.5–185.5 °C; IR (KBr): 3260, 3010, 1732, 1634, 1521, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 363, 1088, 828 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.84 (br, 1H, NH), 9.42 (br, 1H, NH), 7.55–7.54 (m, 2H, ArH), 7.45–7.38 (m, 3H, ArH), 7.35–7.33 (d, J = 9.0 Hz, 2H, ArH), 6.85–6.82 (d, J = 9.0 Hz, 2H, ArH), 4.01–3.99 (m, 1H, CH), 3.70 (s, 3H, OCH3), 3.58–3.56 (m, 2H, CH2N), 3.45–3.44 (m, 2H, NCH2), 2.53–2.22 (m, 2H, CH2CO), 2.02–1.88 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 183.3, 177.7, 168.3, 159.1, 155.8, 142.8, 133.0, 130.1, 129.0, 127.4, 121.4, 114.5, 89.0, 56.0, 41.3, 39.2, 37.8, 37.3, 20.4; HRMS (TOF ES+): m/z calcd for C23H23N3NaO4 [(M + Na)+], 428.1581; found, 428.1579.
363, 1088, 828 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.84 (br, 1H, NH), 9.42 (br, 1H, NH), 7.55–7.54 (m, 2H, ArH), 7.45–7.38 (m, 3H, ArH), 7.35–7.33 (d, J = 9.0 Hz, 2H, ArH), 6.85–6.82 (d, J = 9.0 Hz, 2H, ArH), 4.01–3.99 (m, 1H, CH), 3.70 (s, 3H, OCH3), 3.58–3.56 (m, 2H, CH2N), 3.45–3.44 (m, 2H, NCH2), 2.53–2.22 (m, 2H, CH2CO), 2.02–1.88 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 183.3, 177.7, 168.3, 159.1, 155.8, 142.8, 133.0, 130.1, 129.0, 127.4, 121.4, 114.5, 89.0, 56.0, 41.3, 39.2, 37.8, 37.3, 20.4; HRMS (TOF ES+): m/z calcd for C23H23N3NaO4 [(M + Na)+], 428.1581; found, 428.1579.
2-(8-(4-Fluorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrrolo-[1,2-a]pyrimidin-7-yl)-N-(4-methoxyphenyl)acetamide (3q)
Light yellow solid: mp 225–226 °C; IR (KBr): 3260, 2937, 1732, 1655, 1521, 1088, 1033, 834 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.80 (br, 1H, NH), 9.37 (br, 1H, NH), 7.59–7.56 (m, 2H, ArH), 7.31–7.29 (d, J = 9.0 Hz, 2H, ArH), 7.21–7.19 (m, 2H, ArH), 6.81–6.79 (d, J = 9.0 Hz, 2H, ArH), 3.97–3.95 (m, 1H, CH), 3.68 (s, 3H, OCH3), 3.57–3.53 (m, 2H, CH2N), 3.42–3.41 (m, 2H, NCH2), 2.54–2.23 (m, 2H, CH2CO), 2.00–1.87 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.0, 177.7, 168.3, 163.4 (d, J = 245.0 Hz), 159.3, 155.9, 139.3, 133.0, 129.9, 121.4, 115.9 (d, J = 21.3 Hz), 114.5, 88.9, 56.0, 41.3, 39.2, 37.8, 37.4, 20.4; HRMS (TOF ES+): m/z calcd for C23H22FN3NaO4 [(M + Na)+], 446.1487; found, 446.1484.2-(8-(4-Methoxybenzoyl)-6-oxo-1,2,3,4,6,7-hexahydropyrr-olo[1,2-a]pyrimidin-7-yl)-N-(4-methoxyphenyl)acetamide (3r)
Light yellow solid: mp 165–167 °C; IR (KBr): 3432, 3338, 1727, 1632, 1517, 1250, 1168, 1106, 1031, 841, 768, 616, 535, cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.82 (br, 1H, NH), 9.41 (br, 1H, NH), 7.54–7.52 (d, J = 8.7 Hz, 2H, ArH), 7.33–7.31 (d, J = 9.0 Hz, 2H, ArH), 6.93–6.91 (d, J = 8.7 Hz, 2H, ArH), 6.82–6.80 (d, J = 9.0 Hz, 2H, ArH), 4.03–4.01 (m, 1H, CH), 3.77 (s, 3H, OCH3), 3.68 (s, 3H, OCH3), 3.58–3.56 (m, 2H, CH2N), 3.45–3.41 (m, 2H, NCH2), 2.56–2.31 (m, 2H, CH2CO), 1.98–1.86 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.2, 177.2, 167.9, 160.4, 158.5, 155.4, 134.7, 132.5, 128.8, 120.9, 114.0, 114.0, 113.7, 88.2, 55.5, 41.0, 38.7, 37.3, 36.9, 20.0; HRMS (TOF ES+): m/z calcd for C24H25N3NaO5 [(M + Na)+], 458.1686; found, 458.1686.2-(8-Benzoyl-6-oxo-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyr-imidin-7-yl)-N-ethylacetamide (3s)
Light yellow solid: mp 143.5–144.5 °C; IR (KBr): 3340, 3236, 1734, 1637, 1528, 1445, 1364, 1269, 1162, 1088, 744, 702, 635, 523 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.79 (br, 1H, NH), 7.53–7.47 (m, 2H, ArH), 7.40–7.35 (m, 2H, ArH), 7.33 (br, 1H, NH), 3.87–3.85 (m, 1H, CH), 3.59–3.44 (m, 4H, CH2N), 2.92–2.82 (m, 2H, CH2Me), 2.26–1.97 (m, 2H, CH2CO), 1.94–1.86 (m, 2H, CH2), 0.87–0.84 (m, 3H, CH3); 13C NMR (125 MHz, DMSO-d6): δ = 182.8, 177.2, 168.9, 158.7, 142.3, 129.7, 128.4, 126.9, 88.6, 40.8, 38.7, 37.2, 36.0, 33.5, 19.9, 15.0; HRMS (TOF ES+): m/z calcd for C18H21N3NaO3 [(M + Na)+], 350.1475; found, 350.1475.N-Ethyl-2-(8-(4-fluorobenzoyl)-6-oxo-1,2,3,4,6,7-hexahydro-pyrrolo[1,2-a]pyrimidin-7-yl)acetamide (3t)
Light yellow solid: mp 199–200 °C; IR (KBr): 3324, 3072, 1729, 1631, 1538, 1446, 1372, 1102, 906, 857 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.78 (br, 1H, NH), 7.56–7.54 (m, 2H, ArH), 7.34 (br, 1H, NH), 7.20–7.16 (m, 2H, ArH), 3.85 (m, 1H, CH), 3.54–3.49 (m, 2H, CH2N), 3.40 (m, 2H, NCH2), 2.91–2.80 (m, 2H, CH2Me), 2.30–1.95 (m, 2H, CH2CO), 1.96–1.83 (m, 2H, CH2), 0.86–0.84 (m, 3H, CH3); 13C NMR (125 MHz, DMSO-d6): δ = 181.4, 177.2, 168.8, 162.8 (d, J = 243.8 Hz), 158.8, 138.8, 129.4, 115.3 (d, J = 21.3 Hz), 88.5, 40.8, 38.7, 37.3, 36.0, 33.4, 19.9, 15.0; HRMS (TOF ES+): m/z calcd for C18H20FN3NaO3 [(M + Na)+], 368.1381; found, 368.1379.2-(8-Benzoyl-6-oxo-1,2,3,4,6,7-hexahydropyrrolo[1,2-a]pyr-imidin-7-yl)acetamide (3u)
Light yellow solid: mp 223–224 °C; IR (KBr): 3352, 3154, 1730, 1629, 1525, 1082, 890 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.80 (br, 1H, NH), 7.57–7.47 (m, 2H, ArH), 7.39 (m, 3H, ArH), 6.88 (br, 1H, NH), 6.52 (br, 1H, NH), 3.86–3.84 (m, 1H, CH), 3.55–3.46 (m, 4H, NCH2), 2.25–1.97 (m, 2H, CH2CO), 1.90–1.87 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.7, 177.3, 171.4, 158.7, 142.3, 129.5, 128.5, 127.0, 88.6, 40.7, 38.7, 37.3, 35.6, 19.9; HRMS (TOF ES+): m/z calcd for C16H17N3NaO3 [(M + Na)+], 322.1162; found, 322.1162.2-(9-(4-Fluorobenzoyl)-7-oxo-2,3,4,5,7,8-hexahydro-1H-pyrrolo[1,2-a][1,3]diazepin-8-yl)-N-phenylacetamide (3v)
Light yellow solid: mp 178–180 °C; IR (KBr): 3444, 3314, 1734, 1682, 1619, 1537, 1442, 1090, 853, 760 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.62 (br, 1H, NH), 9.54 (br, 1H, NH), 7.60–7.57 (m, 2H, ArH), 7.41–7.39 (m, 2H, ArH), 7.24–7.19 (m, 4H, ArH), 7.00–6.97 (m, 1H, ArH), 4.07–3.96 (m, 1H, CH), 3.96–3.69 (m, 2H, CH2N), 3.64–3.60 (m, 2H, NCH2), 2.62–2.23 (m, 2H, CH2CO), 1.99–1.82 (m, 4H, CH2CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.0, 168.2, 165.1, 163.9, 162.0, 139.2, 138.6, 129.5, 129.0, 123.4, 119.3, 115.5 (d, J = 20.0 Hz), 90.2, 41.8, 40.9, 40.6, 37.2, 27.0, 24.5; HRMS (TOF ES+): m/z calcd for C23H22FN3NaO3 [(M + Na)+], 430.1537; found, 430.1535.2-(9-(4-Chlorobenzoyl)-7-oxo-2,3,4,5,7,8-hexahydro-1H-pyrrolo[1,2-a][1,3]diazepin-8-yl)-N-phenylacetamide (3w)
Light yellow solid: mp 194.5–195.5 °C; IR (KBr): 3368, 3226, 1736, 1634, 1525, 1438, 1086, 1008, 772 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.62 (br, 1H, NH), 9.56 (br, 1H, NH), 7.55–7.53 (m, 2H, ArH), 7.45–7.43 (m, 2H, ArH), 7.39–7.38 (m, 2H, ArH), 7.24–7.21 (m, 2H, ArH), 6.99–6.9 (m, 2H, ArH), 4.00–3.98 (m, 1H, CH), 3.91–3.75 (m, 2H, CH2N), 3.61–3.60 (m, 2H, NCH2), 2.62–2.22 (m, 2H, CH2CO), 1.92–1.90 (m, 4H, CH2CH2); 13C NMR (125 MHz, DMSO-d6): δ = 181.8, 178.4, 168.2, 165.2, 140.8, 139.2, 134.3, 129.0, 129.0, 128.7, 128.7, 123.4, 119.4, 90.4, 41.8, 40.7, 37.2, 27.0, 24.5; HRMS (TOF ES+): m/z calcd for C23H22ClN3NaO3 [(M + Na)+], 446.1242; found, 446.1243.2-(9-(4-Methylbenzoyl)-7-oxo-2,3,4,5,7,8-hexahydro-1H-pyrrolo[1,2-a][1,3]diazepin-8-yl)-N-phenylacetamide (3x)
Light yellow solid: mp 197–198 °C; IR (KBr): 3248, 2917, 1737, 1683, 1619, 1536, 1440, 1053, 898, 756 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.64 (br, 1H, NH), 9.56 (br, 1H, NH), 7.45–7.40 (m, 4H, ArH), 7.24–7.19 (m, 4H, ArH), 6.99–6.96 (m, 1H, ArH), 4.05–4.03 (m, 1H, CH), 3.91–3.72 (m, 2H, CH2N), 3.66–3.48 (m, 2H, NCH2), 2.59–2.26 (m, 2H, CH2CO), 2.32 (s, 3H, CH3), 2.00–1.80 (m, 4H, CH2CH2); 13C NMR (125 MHz, DMSO-d6): δ = 183.4, 178.5, 168.3, 164.9, 139.3, 129.1, 128.9, 128.9, 127.1, 123.3, 119.3, 119.3, 90.3, 41.9, 41.0, 40.7, 37.2, 27.1, 24.6, 21.4; HRMS (TOF ES+): m/z calcd for C24H25N3NaO3 [(M + Na)+], 426.1788; found, 426.1785.N-Benzyl-2-(9-(4-fluorobenzoyl)-7-oxo-2,3,4,5,7,8-hexahy-dro-1H-pyrrolo[1,2-a][1,3]diazepin-8-yl)acetamide (3y)
Light yellow solid: mp 110-112 °C; IR (KBr): 3389, 3064, 1731, 1619, 1537, 1442, 1078, 837 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.67 (br, 1H, NH), 7.93 (br, 1H, NH), 7.60–7.68 (m, 2H, ArH), 7.31–7.28 (m, 2H, ArH), 7.22–7.18 (m, 3H, ArH), 7.09–7.07 (m, 2H, ArH), 4.21–3.91 (m, 2H, CH2Ph), 3.94–3.91 (m, 1H, CH), 3.89–3.68 (m, 2H, CH2N), 3.58–3.55 (m, 2H, NCH2), 2.49–2.08 (m, 2H, CH2CO), 1.97–1.79 (m, 4H, CH2CH2); 13C NMR (125 MHz, DMSO-d6): δ = 182.0, 178.5, 169.2, 165.3, 162.4 (d, J = 245.0 Hz), 139.7, 138.6, 129.5, 128.5, 127.2, 126.9, 115.4 (d, J = 21.3 Hz), 90.2, 42.1, 41.9, 41.0, 36.1, 27.0, 24.5; HRMS (TOF ES+): m/z calcd for C24H24FN3NaO3 [(M + Na)+], 444.1693; found, 444.1694.N-Benzyl-2-(9-(4-methylbenzoyl)-7-oxo-2,3,4,5,7,8-hexahy-dro-1H-pyrrolo[1,2-a][1,3]diazepin-8-yl)acetamide (3z)
Light yellow solid: mp 110.5–112 °C; IR (KBr): 3289, 2921, 1734, 1669, 1618, 1527, 1440, 1048, 738 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.68 (br, 1H, NH), 7.92 (br, 1H, NH), 7.45–7.44 (m, 2H, ArH), 7.31–7.27 (m, 2H, ArH), 7.22–7.18 (m, 3H, ArH), 7.12–7.09 (m, 2H, ArH), 4.21–3.99 (m, 2H, CH2Ph), 3.92–3.62 (m, 2H, CH2N), 3.59–3.54 (m, 1H, CH), 3.44–2.55 (m, 2H, NCH2), 2.45–2.11 (m, 2H, CH2CO), 2.33 (s, 3H, CH3), 1.88–1.784 (m, 4H, CH2CH2); 13C NMR (125 MHz, DMSO-d6): δ = 183.3, 178.5, 169.3, 165.1, 139.7, 139.4, 139.1, 129.0, 128.5, 127.2, 126.9, 90.3, 56.4, 42.1, 41.9, 40.7, 36.1, 27.1, 24.6, 21.4; HRMS (TOF ES+): m/z calcd for C25H27N3NaO3 [(M + Na)+], 440.1945; found, 440.1943.2-(7-Benzoyl-5-oxo-2,3,5,6-tetrahydro-1H-pyrrolo[1,2-a]-imidazol-6-yl)-N-phenylacetamide (3a′)
Light yellow solid: mp 139–142.5 °C; IR (KBr): 3257, 3060, 1727, 1635, 1525, 1442, 1112, 742 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.55 (br, 1H, NH), 9.46 (br, 1H, NH), 7.45–7.43 (m, 2H, ArH), 7.39–7.32 (m, 3H, ArH), 7.28–7.24 (m, 4H, ArH), 7.04–7.01 (m, 1H, ArH), 3.92–3.88 (m, 1H, CH), 3.80–3.61 (m, 4H, CH2CH2), 2.97–2.50 (m, 2H, CH2CO); 13C NMR (125 MHz, DMSO-d6): δ = 189.7, 172.7, 166.4, 154.5, 142.4, 139.2, 129.1, 129.0, 128.5, 126.5, 123.7, 119.8, 84.7, 43.1, 41.9, 41.0, 36.5; HRMS (TOF ES+): m/z calcd for C21H19N3NaO3 [(M + Na)+], 384.1319; found, 384.1320.2-(7-(4-Methoxybenzoyl)-5-oxo-2,3,5,6-tetrahydro-1H-pyrrolo[1,2-a]imidazol-6-yl)-N-phenylacetamide (3b′)
Light yellow solid: mp 122–125 °C; IR (KBr): 3416, 3305, 1690, 1614, 1496, 1443, 1170, 1023, 841 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.66 (br, 1H, NH), 9.24 (br, 1H, NH), 7.47–7.45 (m, 2H, ArH), 7.29–7.26 (m, 4H, ArH), 7.04–7.02 (m, 1H, ArH), 6.91–6.90 (m, 2H, ArH), 3.89–3.86 (m, 1H, CH), 3.76 (s, 3H, OCH3), 3.71–3.70 (m, 4H, CH2CH2), 2.95–2.58 (m, 2H, CH2CO); 13C NMR (125 MHz, DMSO-d6): δ = 189.3, 172.7, 167.5, 160.2, 157.2, 139.3, 134.6, 129.1, 128.6, 123.7, 119.7, 113.7, 84.7, 55.6, 43.0, 41.9, 41.1, 36.3; HRMS (TOF ES+): m/z calcd for C22H21N3NaO4 [(M + Na)+], 414.1424; found, 414.1427.2-(7-Benzoyl-5-oxo-2,3,5,6-tetrahydro-1H-pyrrolo[1,2-a]-imidazol-6-yl)-N-(4-fluorophenyl)acetamide (3c′)
Light yellow solid: mp 131–132.5 °C; IR (KBr): 3499, 3400, 1686, 1620, 1508, 1102, 1013, 849 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.65 (br, 1H, NH), 9.48 (br, 1H, NH), 7.47–7.44 (m, 2H, ArH), 7.36–7.35 (m, 3H, ArH), 7.24–7.23 (m, 2H, ArH), 7.13–7.09 (m, 2H, ArH), 3.92–3.89 (m, 1H, CH), 3.77–3.57 (m, 4H, CH2CH2), 2.98–2.55 (m, 2H, CH2CO); 13C NMR (125 MHz, DMSO-d6): δ = 189.7, 172.6, 167.4, 159.4, 157.5, 142.4, 135.6, 129.1, 128.5, 126.4, 121.5, 115.6 (d, J = 21.3 Hz), 84.6, 43.1, 41.8, 40.9, 36.5; HRMS (TOF ES+): m/z calcd for C21H18FN3NaO3 [(M + Na)+], 402.1224; found, 402.1226.2-(7-Benzoyl-5-oxo-2,3,5,6-tetrahydro-1H-pyrrolo[1,2-a]-imidazol-6-yl)-N-(4-methoxyphenyl)acetamide (3d′)
Light yellow solid: mp 181–182 °C; IR (KBr): 3264, 3072, 2958, 1689, 1624, 1504, 1025, 833, 702 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.49 (br, 1H, NH), 9.41 (br, 1H, NH), 7.36–7.33 (m, 5H, ArH), 7.25–7.22 (m, 2H, ArH), 6.85–6.83 (m, 2H, ArH), 3.92–3.85 (m, 1H, CH), 3.78–3.71 (m, 2H, CH2N), 3.70 (s, 3H, OCH3), 3.68–3.53 (m, 2H, NCH2), 2.95–2.54 (m, 2H, CH2CO); 13C NMR (125 MHz, DMSO-d6): δ = 189.7, 172.2, 167.5, 157.5, 155.6, 142.5, 132.3, 129.1, 128.4, 126.4, 121.3, 114.1, 84.8, 55.5, 43.1, 41.8, 40.8, 36.6; HRMS (TOF ES+): m/z calcd for C22H21N3NaO4 [(M + Na)+], 414.1424; found, 414.1423.N-Benzyl-2-(7-(4-methoxybenzoyl)-5-oxo-2,3,5,6-tetrahyd-ro-1H-pyrrolo[1,2-a]imidazol-6-yl)acetamide (3e′)
Light yellow solid: mp 191–193 °C; IR (KBr): 3309, 2925, 1691, 1625, 1498, 1450, 1021, 846, 702 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 9.44 (br, 1H, NH), 8.09 (br, 1H, NH–Bn), 7.32–7.31 (m, 2H, ArH), 7.25–7.23 (m, 3H, ArH), 7.21–7.20 (m, 2H, ArH), 6.82–6.80 (m, 2H, ArH), 4.26–4.11 (m, 2H, CH2Ph), 3.92–3.82 (m, 1H, CH), 3.75 (s, 3H, OCH3), 3.67–3.51 (m, 4H, CH2CH2), 2.86–2.55 (m, 2H, CH2CO); 13C NMR (125 MHz, DMSO-d6): δ = 189.1, 173.6, 167.8, 160.0, 139.8, 134.6, 128.6, 128.6, 127.5, 127.1, 113.5, 84.7, 55.5, 43.1, 42.6, 41.8, 40.1, 36.6; HRMS (TOF ES+): m/z calcd for C23H23N3NaO4 [(M + Na)+], 428.1581; found, 428.1579.2-(7-Benzoyl-5-oxo-2,3,5,6-tetrahydro-1H-pyrrolo[1,2-a]-imidazol-6-yl)-N-ethylacetamide (3f′)
Light yellow solid: mp 230.5–231.5 °C; IR (KBr): 3110, 2952, 1695, 1590, 1529, 1458, 1123, 984, 774 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.18 (br, 1H, NH), 7.29–7.25 (m, 3H, ArH), 7.18–7.14 (m, 2H, ArH), 6.82 (br, 1H, NH), 3.69–3.65 (m, 1H, CH), 3.53–3.47 (m, 4H, CH2CH2), 3.17–3.12 (m, 2H, CH2Me), 2.84–2.41 (m, 2H, CH2CO), 0.93–0.86 (m, 3H, CH3); 13C NMR (125 MHz, DMSO-d6): δ = 189.0, 179.8, 176.4, 165.8, 144.4, 128.7, 127.2, 85.1, 43.7, 43.7, 40.0, 37.0, 33.7, 13.5; HRMS (TOF ES+): m/z calcd for C17H20N3O3 [(M + H)+], 314.1499; found, 314.1493.2-(7-(4-Chlorobenzoyl)-5-oxo-2,3,5,6-tetrahydro-1H-pyrr-olo[1,2-a]imidazol-6-yl)-N-ethylacetamide (3g′)
Light yellow solid: mp 221–223 °C; IR (KBr): 3395, 3273, 1690, 1589, 1523, 1401, 1127, 841 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.12 (br, 1H, NH), 7.35–7.33 (m, 2H, ArH), 7.21–7.17 (m, 2H, ArH), 6.86 (br, 1H, NH), 3.67–3.50 (m, 4H, CH2CH2), 3.17–3.14 (m, 1H, CH), 2.86–2.45 (m, 2H, CH2CO), 2.52–2.48 (m, 2H, CH2Me), 0.90–0.86 (m, 3H, CH3); 13C NMR (125 MHz, DMSO-d6): δ = 187.1, 179.1, 175.9, 165.3, 142.6, 132.7, 128.8, 128.2, 84.8, 43.1, 43.1, 40.0, 36.5, 33.2, 12.9; HRMS (TOF ES+): m/z calcd for C17H18ClN3NaO3 [(M + Na)+], 370.0929; found, 370.0928.N-Ethyl-2-(7-(4-methoxybenzoyl)-5-oxo-2,3,5,6-tetrahydro-1H-pyrrolo[1,2-a]imidazol-6-yl)acetamide (3h′)
Light yellow solid: mp 183–186 °C; IR (KBr): 3195, 2966, 1693, 1591, 1522, 1460, 1120, 1031, 825 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 10.19 (br, 1H, NH), 7.14 (d, J = 7.2 Hz, 2H, ArH), 6.84 (d, J = 8.1 Hz, 2H, ArH), 6.73 (br, 1H, NH), 3.85–3.81 (m, 1H, CH), 3.73 (s, 3H, OCH3), 3.50–3.46 (m, 4H, CH2CH2), 3.23–3.19 (m, 2H, CH2Me), 2.85–2.45 (m, 2H, CH2CO), 0.94–0.89 (m, 3H, CH3); 13C NMR (125 MHz, DMSO-d6): δ = 188.4, 179.4, 176.0, 165.2, 159.3, 136.3, 128.4, 113.5, 113.5, 84.8, 55.4, 43.1, 43.1, 36.5, 33.2, 13.0; HRMS (TOF ES+): m/z calcd for C18H22N3O4 [(M + H)+], 344.1605; found, 344.1607.Acknowledgements
We gratefully acknowledge the financial support from the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT13095), the National Natural Science Foundation of China (nos. U1202221, 21162037, 21262042, 21362042 and 81160384), the Reserve Talent Foundation of Yunnan Province for Middle-aged and Young Academic and Technical Leaders (no. 2012HB001), and the Scientific Research Fund of Yunnan Provincial Education Department (2013Y363).References
- For selected examples see: (a) R. H. Feling, G. O. Buchana, T. J. Mincer, C. A. Kauffman, P. R. Jensen and W. Fenical, Angew. Chem., Int. Ed., 2003, 42, 355 CrossRef CAS PubMed; (b) H. J. Shin, T. S. Kim, H.-S. Lee, J. Y. Park, I.-K. Choi and H. J. Kwon, Phytochemistry, 2008, 69, 2363 CrossRef CAS PubMed; (c) P. A. Reddy, B. C. H. Hsiang, T. N. Latifi, M. W. Hill, K. E. Woodward, S. M. Rothman, J. A. Ferrendelli and D. F. Covey, J. Med. Chem., 1996, 39, 1898 CrossRef CAS PubMed; (d) J. J.-W. Duan, L. Chen, Z. R. Wasserman, Z. Lu, R.-Q. Liu, M. B. Covington, M. Qian, K. D. Hardman, R. L. Magoda, R. C. Newton, D. D. Christ, R. R. Wexler and C. P. Decicco, J. Med. Chem., 2002, 45, 4954 CrossRef CAS PubMed; (e) E. O. Onyango, J. Tsurumoto, N. Imai, K. Takahashi, J. Ishihara and S. Hatakeyama, Angew. Chem., Int. Ed., 2007, 46, 6703 CrossRef CAS PubMed; (f) S. Tekkam, M. A. Alam, S. C. Jonnalagadda and V. R. Mereddy, Chem. Commun., 2011, 47, 3219 RSC; (g) N. Kogure, N. Ishii, M. Kitajima, S. Wongseripipatana and H. Takayama, Org. Lett., 2006, 8, 3085 CrossRef CAS PubMed; (h) T. Heinrich, F. Zenke, M. Calderini, D. Musil, US 2013/0296274 A1, 2013.
- (a) R. Grote, A. Zeeck, J. Stümpfel and H. Zähner, Liebigs Ann. Chem., 1990, 525 CrossRef CAS; (b) G. H. Hakimelahi, A. A. Moosavi-Movahedi, S.-C. Tsay, F.-Y. Tsai, J. D. Wright, T. Dudev, S. Hakimelahi and C. Lim, J. Med. Chem., 2000, 43, 3632 CrossRef CAS PubMed.
- G. J. Morriello, R. J. Devita, S. G. Mills, J. R. Young, P. Lin, G. Doss, G. G. Chicchi, J. DeMartina, M. M. Kurtz, K.-L. C. Tsao, E. Carlson, K. Townson, A. Wheeldon, S. Boyce, N. Collinson, N. Rupniak and S. Moore, Bioorg. Med. Chem., 2008, 16, 2156 CrossRef CAS PubMed.
- (a) T. A. M. Gulder and B. S. Moore, Angew. Chem., Int. Ed., 2010, 49, 9346 CrossRef CAS PubMed; (b) W. M. Kazmierski, W. Andrews, E. Furfine, A. Spaltenstein and L. Wright, Bioorg. Med. Chem. Lett., 2004, 14, 5689 CrossRef CAS PubMed.
- K. D. Sarma, J. Zhang, Y. Huang and J. G. Davidson, Eur. J. Org. Chem., 2006, 3730 CrossRef.
- P. Y. Ng, Y.-C. Tang, W. M. Knosp, H. S. Stadler and J. T. Shaw, Angew. Chem., Int. Ed., 2007, 46, 5352 CrossRef CAS PubMed.
- (a) I. Tellitu, S. Serna, M. T. Herrero, I. Moreno, E. Dominguez and R. SanMartin, J. Org. Chem., 2007, 72, 1526 CrossRef CAS PubMed; (b) D. Yang, G.-Y. Lian, H.-F. Yang, J.-D. Yu, D.-W. Zhang and X. Gao, J. Org. Chem., 2009, 74, 8610 CrossRef CAS PubMed; (c) M. Blangetti, A. Deagostino, G. Gervasio, D. Marabello, C. Prandi and P. Venturelloa, Org. Biomol. Chem., 2011, 9, 2535 RSC.
- (a) P. Y. Ng, C. E. Masse and J. T. Shaw, Org. Lett., 2006, 8, 3999 CrossRef CAS PubMed; (b) O. Pattawong, D.-Q. Tan, J. C. Fettinger, J. T. Shaw and P. H.-Y. Cheong, Org. Lett., 2013, 15, 5130 CrossRef CAS PubMed.
- (a) X.-Y. Liu, C.-H. Li and C.-M. Che, Org. Lett., 2006, 8, 2707 CrossRef CAS PubMed; (b) C.-Y. Zhou and C.-M. Che, J. Am. Chem. Soc., 2007, 129, 5828 CrossRef CAS PubMed; (c) C. Shu, M.-Q. Liu, S.-S. Wang, L. Li and L.-W. Ye, J. Org. Chem., 2013, 78, 3292 CrossRef CAS PubMed.
- (a) C. H. Yoon, A. Nagle, C. Chen, D. Gandhi and K. W. Jung, Org. Lett., 2003, 5, 2259 CrossRef CAS PubMed; (b) T. K. Hyster, K. E. Ruhl and T. Rovis, J. Am. Chem. Soc., 2013, 135, 5364 CrossRef CAS PubMed.
- (a) W. V. Brabandt and N. D. Kimpe, J. Org. Chem., 2005, 70, 3369 CrossRef PubMed; (b) W. V. Brabandt and N. D. Kimpe, J. Org. Chem., 2005, 70, 8717 CrossRef PubMed; J.-H. Park, J.-R. Ha, S.-J. Oh, J.-A. Kim, D.-S. Shin, T.-J. Won, Y.-F. Lamb and C. Ahna, Tetrahedron Lett., 2005(46), 1755 Search PubMed.
- (a) Reviews see: K.-M. Wang, S.-J. Yan and J. Lin, Eur. J. Org. Chem., 2014, 1129 CrossRef CAS . For recent examples see:; (b) F.-C. Yu, Z.-Q. Chen, X.-P. Hao, S.-J. Yan, R. Huang and J. Lin, RSC Adv., 2014, 4, 6110 RSC; (c) X.-B. Chen, Z.-C. Liu, L. Fang, S.-J. Yan and J. Lin, ACS Sustainable Chem. Eng., 2014, 2, 1155 CrossRef CAS; (d) Y.-C. Zhang, Z.-C. Liu, R. Yang, J.-H. Zhang, S.-J. Yan and J. Lin, Org. Biomol. Chem., 2013, 11, 7276 RSC; (e) X.-B. Chen, X.-Y. Wang, D.-D. Zhu, S.-J. Yan and J. Lin, Tetrahedron, 2014, 70, 1047 CrossRef CAS PubMed; (f) X.-B. Chen, D.-D. Zhu, X.-Y. Wang, S.-J. Yan and J. Lin, Tetrahedron, 2013, 69, 9224 CrossRef CAS PubMed.
- (a) C. Huang, S.-J. Yan, X.-H. Zeng, X.-Y. Dai, Y. Zhang, C. Qing and J. Lin, Eur. J. Med. Chem., 2011, 46, 1172 CrossRef CAS PubMed; (b) S. J. Yan, Y.-J. Liu, Y.-L. Chen, L. Liu and J. Lin, Bioorg. Med. Chem. Lett., 2010, 20, 5225 CrossRef CAS PubMed.
- (a) F.-C. Yu, Z.-Q. Chen, X.-P. Hao, X.-Y. Jiang, S.-Y. Yan and J. Lin, RSC Adv., 2013, 3, 13183 RSC; (b) S.-J. Yan, C. Huang, C.-X. Su, Y.-F. Ni and J. Lin, J. Comb. Chem., 2010, 12, 91 CrossRef CAS PubMed.
- S.-J. Yan and J. Lin, Chin. J. Chem., 2010, 30, 465 CAS.
- (a) P. V. Kattamuri, T. Ai, S. Pindi, Y.-W. Sun, P. Gu, M. Shi and G. Li, J. Org. Chem., 2011, 76, 2792 CrossRef CAS PubMed; (b) A. Kattuboina and G. Li, Tetrahedron Lett., 2008, 49, 1573 CrossRef CAS PubMed; (c) P. Kaur, S. Pindi, W. Wever, T. Rajale and G. Li, J. Org. Chem., 2010, 75, 5144 CrossRef CAS PubMed; (d) S. Pindi, P. Kaur, G. Shakya and G. Li, Chem. Biol. Drug Des., 2011, 77, 20 CrossRef CAS PubMed.
- C.-Y. Yu, P.-H. Yang, M.-X. Zhao and Z.-T. Huang, Synlett, 2006, 12, 1835 CrossRef PubMed.
- (a) Z.-T. Huang and M.-X. Wang, Synthesis, 1992, 12, 1273 CrossRef PubMed; (b) Z.-J. Li and D. Charles, Synth. Commun., 2001, 31, 527 CrossRef CAS PubMed.
- Z.-C. Chen, Z.-G. Le, Z.-C. Chen, Y. Hu, Z.-G. Le and Q.-G. Zheng, Synthesis, 2004, 7, 995 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 998644 (3t). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra03863k |
| This journal is © The Royal Society of Chemistry 2014 |