Reduction-triggered formation of EFK8 molecular hydrogel for 3D cell culture†
Abstract
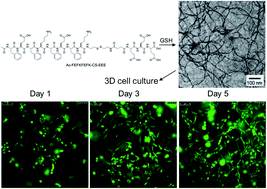
We report here a biocompatible strategy of EFK8-based molecular hydrogel formation via disulfide bond reduction in cell culture media under neutral conditions, which presents a promising approach in three-dimensional (3D) cell culture.

 Please wait while we load your content...
Please wait while we load your content...