A turn-on fluorescent probe for imaging lysosomal hydrogen sulfide in living cells†
Qinglong Qiaoab,
Miao Zhaob,
Haijing Langb,
Deqi Maoab,
Jingnan Cui*a and
Zhaochao Xu*ab
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116012, China. E-mail: zcxu@dicp.ac.cn; jncui@dlut.edu.cn; Fax: +86-411-84379648; Tel: +86-411-84379648
bKey Laboratory of Separation Science for Analytical Chemistry of CAS, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 3rd June 2014
Abstract
Hydrogen sulfide (H2S) is an endothelial gasotransmitter which has been extensively studied recently in various physiological processes. H2S can induce lysosomal membrane destabilization leading to an autophagic event of precipitation apoptosis coupled with calpain activation, thus ensuring cellular demise. In this study, we developed a lysosome-targetable fluorescent probe for the recognition of H2S with considerable fluorescence enhancement. Through introducing a lysosome-targetable group 4-(2-aminoethyl)-morpholine into the H2S probe N-imide termus of 4-azide-1,8-naphthalimide, the new compound Lyso-AFP can recognize H2S in lysosomes. This probe emerges as a more biocompatible analysis tool with low poison by-product than reported H2S fluorescent probes.
Introduction
Fluorescence imaging in living cells is a powerful technique to study biological systems in vivo.1,2 By attaching a sub-cellular organelle specific group, fluorescent probes are able to detect target analytes and reveal a diverse range of physical/chemical properties in specific regions of a cell.3–5 Lysosomes are spherical-shaped, catabolic organelles with an acidic interior (pH 4.0–6.0). They are vital for degradation and recycling of macromolecules delivered by phagocytosis, endocytosis, and autophagy. Lysosomes were considered merely to be cellular waste bags for a long time. Nowadays, lysosomes are recognized as advanced organelles involved in many cellular processes and are considered crucial regulators of cell homeostasis.6,7 Evidences have shown that lysosomes are related to the pathogenesis of diseases such as storage disorders, cancer, neurodegenerative disorders, and cardiovascular diseases.6 So real-time detection and imaging of lysosomal analytes would aid the understanding of intracellular reaction kinetics and mechanisms, and further assist the development of diagnostic and treatment strategies. In recent years, some fluorescent probes have been reported to stain lysosomes8–13 or image lysosomal pH,14–16 Ca2+,17 Zn2+,18–20 Cu2+,21,22 NO,23 H2O2,24 legumain,25 viscosity,26 and phospholipase A2 activity.27Hydrogen sulfide (H2S), a well known pungent gas, is generated endogenously in mammalian tissues from the amino acids cysteine and homocysteine by three enzymes including cystathionine-β-lyase (CSE), cystathionine-γ-synthetase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST).28 Nowadays, H2S has been considered as a crucial signal molecule in nervous system, cardiovascular system, and inflammatory system. In the nervous system, H2S has been found to modulate neuronal transmission by facilitating the induction of hippocampal long term potential (LTP).29 In the cardiovascular system, H2S can relax muscle and regulate blood pressure.29 H2S is also believed to be related with some diseases like Alzheimer's disease,30 Down's syndrome,31 diabetes32 and liver cirrhosis.33 Furthermore, H2S also functions in lysosome organelles. H2S can induce cell death in association with the activation of calpain proteases and lysosomal destabilization along with the release of lysosomal proteases.34 Therefore, high sensitive and selective techniques for detecting H2S in lysosomes seem to be great valuable.
In our previous work, we reported the first lysosome-targetable fluorescent probe Lyso-NHS for imaging H2S in living cells based on the thiolysis of dinitrophenyl ether.35 In consideration of potential toxicity of the leaving dinitrophenyl thiol ether to biological system, a much more biocompatible fluorescent probe for lysosomal H2S imaging is desired. Chang et al.36 and Wang37 et al. pioneered an approach of using the reduction of azide with H2S to amine to sense H2S, which releases a much non-cytotoxic N2 as the byproduct. This approach has been expanded to design various fluorescent probes for H2S by altering fluorophores.38–43 In this work, we introduced a lysosome-targetable group 4-(2-aminoethyl)-morpholine23 into the N-imide termus of 4-azide-1,8-naphthalimide to yield the fluorescent probe Lyso-AFP (Scheme 1), and studied its properties in lysosomal H2S imaging.
Experimental section
Materials and methods
Unless otherwise noted, materials were obtained from Aldrich and were used without further purification. The synthesis of compound N-(morpholinoethylamino)-4-bromo-1,8-naphthalimide (3) was according to the published procedure.35 Melting points were measured using a Büchi 530 melting point apparatus. 1H NMR and 13C NMR spectra were recorded using Bruker 400 MHz. Chemical shifts were given in ppm and coupling constants in Hz. UV-Vis absorption spectra were obtained on Agilent Cary 60 UV-Vis Spectrophotometer. Fluorescence emission spectra were obtained using Cary Eclipse Fluorescence Spectrophotometer.Synthesis and characterization of Lyso-AFP
A solution of sodium azide 350 mg (5.4 mmol) in 5 mL water was added dropwise into the solution of compound 3 (2.0 g, 5.1 mmol) in 30 mL DMF. The reaction mixture was stirred at 100 °C for 8 hours. Then the mixture was added into ice water. Yellow solid was collected and dried in a vacuum drying oven, which was purified by silica gel column chromatography (CH2Cl2–MeOH = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound Lyso-AFP (1.7 g) in 90% yield. Mp: 144–146 °C. 1H NMR (400 MHz, CDCl3) δ 8.62 (d, J = 12.0 Hz, 1H), 8.57 (d, J = 8.0 Hz, 1H), 8.43 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.47 (t, J = 8.0 Hz, 1H), 4.33 (t, J = 6.0 Hz, 2H), 3.68 (t, J = 4.0 Hz, 4H), 2.70 (t, J = 6.0 Hz, 2H), 2.59 (J = 4.0 Hz, 4H). 13C NMR (100 MHz, CDCl3) δ 164.0, 163.6, 143.5, 132.2, 131.7, 129.2, 128.8, 126.9, 124.4, 122.6, 118.9, 114.7, 67.1, 56.2, 53.8, 37.3. HRMS (ESI) calcd for C18H18N5O3 [MH+] 352.1404, found 352.1419.
1) to afford compound Lyso-AFP (1.7 g) in 90% yield. Mp: 144–146 °C. 1H NMR (400 MHz, CDCl3) δ 8.62 (d, J = 12.0 Hz, 1H), 8.57 (d, J = 8.0 Hz, 1H), 8.43 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.47 (t, J = 8.0 Hz, 1H), 4.33 (t, J = 6.0 Hz, 2H), 3.68 (t, J = 4.0 Hz, 4H), 2.70 (t, J = 6.0 Hz, 2H), 2.59 (J = 4.0 Hz, 4H). 13C NMR (100 MHz, CDCl3) δ 164.0, 163.6, 143.5, 132.2, 131.7, 129.2, 128.8, 126.9, 124.4, 122.6, 118.9, 114.7, 67.1, 56.2, 53.8, 37.3. HRMS (ESI) calcd for C18H18N5O3 [MH+] 352.1404, found 352.1419.
Synthesis and characterization of compound 1
Lyso-AFP (100 mg, 0.28 mmol) was added to a round bottom flask under argon and dissolved in 50 mL acetonitrile. Then NaHS (24 mg, 0.43 mmol) was added slowly and the mixture was allowed to stir at room temperature for 24 h. The solvent was removed under reduced pressure and the resulted brown solid was purified by silica gel column chromatography (CH2Cl2–MeOH = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 1 in 83% yield. 1H NMR (400 MHz, DMSO) δ 8.61 (d, J = 8.4 Hz, 1H), 8.43 (d, J = 8.4 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.76–7.59 (m, 1H), 7.42 (s, 2H), 6.85 (d, J = 8.4 Hz, 1H), 4.15 (t, J = 7.0 Hz, 2H), 3.53 (t, J = 8.0 Hz, 4H), 2.53 (t, J = 7.0 Hz, 2H), 2.46 (t, J = 8.4 Hz, 4H). HRMS (ESI) calcd for C18H20N3O3 [MH+] 326.1499, found 326.1524.
1) to afford compound 1 in 83% yield. 1H NMR (400 MHz, DMSO) δ 8.61 (d, J = 8.4 Hz, 1H), 8.43 (d, J = 8.4 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.76–7.59 (m, 1H), 7.42 (s, 2H), 6.85 (d, J = 8.4 Hz, 1H), 4.15 (t, J = 7.0 Hz, 2H), 3.53 (t, J = 8.0 Hz, 4H), 2.53 (t, J = 7.0 Hz, 2H), 2.46 (t, J = 8.4 Hz, 4H). HRMS (ESI) calcd for C18H20N3O3 [MH+] 326.1499, found 326.1524.
Culture of Hela cells and fluorescent imaging
Hela was cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% FBS (fetal bovine serum) in an atmosphere of 5% CO2 and 95% air at 37 °C. The cells were seeded in 24-well flat-bottomed plates and then incubated for 48 h at 37 °C under 5% CO2. Lyso-AFP (5 μM) was then added to the cells and incubation for 30 min followed. Neutral Red (NR) (2 μM) was next added to co-stain the cells for 10 min. Then, the cells were washed three times with phosphate-buffered saline (PBS). Fluorescence imaging was observed under a confocal microscopy (Olympus FV1000) with a 60× objective lens.Results and discussion
Effect of pH on the fluorescence of Lyso-AFP
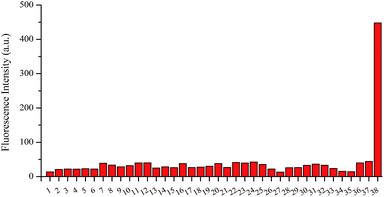
In lysosomes, in order to maintain the pH in range of 4.0–6.0, Vacuolar H+-ATPases are usually responsible for transport of protons.44 So, to monitor H2S in lysosomes, the probe should remain stable in acidic environment with no fluorescence response. Firstly, we investigated behavior of Lyso-AFP in a wide range of pH values in acetonitrile–water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) solution (Fig. 1). What we can see from the fluorescence spectrum of Lyso-AFP is that the probe exhibited a weak emission band with a maximum at 535 nm. Hence, the stable fluorescence of Lyso-AFP in the pH range 3.0–12.0 can provide its application in monitoring intracellular H2S without being affected by changes in physiological pH values.
50) solution (Fig. 1). What we can see from the fluorescence spectrum of Lyso-AFP is that the probe exhibited a weak emission band with a maximum at 535 nm. Hence, the stable fluorescence of Lyso-AFP in the pH range 3.0–12.0 can provide its application in monitoring intracellular H2S without being affected by changes in physiological pH values.
 | ||
| Fig. 1 Influence of pH on the fluorescence of Lyso-AFP in aqueous solution. Excitation wavelength is 426 nm [Lyso-AFP] = 10 μM. | ||
Characterization of fluorescent probe Lyso-AFP for H2S
Firstly, the absorption spectrum of Lyso-AFP in aqueous solution (CH3CN–HEPES = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, pH = 7.4) shows an absorption band at 370 nm. When we add NaHS (10 μM) to the solution of the probe, the band centered at 370 nm displays sharp decrease in absorbance along with the appearance of a new absorption band at longer wavelength (426 nm) (Fig. 2) which is visible to the naked eye with a clear colour change from colourless to pale yellow. And, it indicates if the concentration of NaHS reach 20 equiv., the reaction can be completely finished in minutes.
5, pH = 7.4) shows an absorption band at 370 nm. When we add NaHS (10 μM) to the solution of the probe, the band centered at 370 nm displays sharp decrease in absorbance along with the appearance of a new absorption band at longer wavelength (426 nm) (Fig. 2) which is visible to the naked eye with a clear colour change from colourless to pale yellow. And, it indicates if the concentration of NaHS reach 20 equiv., the reaction can be completely finished in minutes.
 | ||
Fig. 2 UV-Vis absorption spectra of 10 μM compound Lyso-AFP in the presence of 0–20 equiv. of H2S in aqueous solution (CH3CN–HEPES = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, pH = 7.4). 50, pH = 7.4). | ||
We then tested the fluorescence properties of Lyso-AFP for sensing H2S in aqueous solution (CH3CN–HEPES = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, pH = 7.4) (Fig. 3). Spectra were recorded after the addition of H2S from 0 to 40 min, and the results showed that the reaction was completed within 30 min (Fig. 3a and b). Notably, the background fluorescence of Lyso-AFP is very weak (Φ = 0.012), and within minutes a high fluorescence (Φ = 0.263) increase is observed which signals the reaction of Lyso-AFP with H2S (Fig. 3b); therefore, the timescale may allow Lyso-AFP to sense H2S in real-time intracellular imaging. Furthermore, when H2S was added progressively from 0 equiv. to 30 equiv. to the solution of Lyso-AFP, the fluorescence intensity at 535 nm was dramatically increased due to the reduction of azide group to amine by H2S (Scheme 1). From Fig. 3d, it was also found that if the concentration of NaHS was over 20 equiv., the reaction can be completed. Therefore, we used 20 equiv. of H2S to examine the performance of Lyso-AFP in all following experiments.
50, pH = 7.4) (Fig. 3). Spectra were recorded after the addition of H2S from 0 to 40 min, and the results showed that the reaction was completed within 30 min (Fig. 3a and b). Notably, the background fluorescence of Lyso-AFP is very weak (Φ = 0.012), and within minutes a high fluorescence (Φ = 0.263) increase is observed which signals the reaction of Lyso-AFP with H2S (Fig. 3b); therefore, the timescale may allow Lyso-AFP to sense H2S in real-time intracellular imaging. Furthermore, when H2S was added progressively from 0 equiv. to 30 equiv. to the solution of Lyso-AFP, the fluorescence intensity at 535 nm was dramatically increased due to the reduction of azide group to amine by H2S (Scheme 1). From Fig. 3d, it was also found that if the concentration of NaHS was over 20 equiv., the reaction can be completed. Therefore, we used 20 equiv. of H2S to examine the performance of Lyso-AFP in all following experiments.
In addition, compound 1 was synthesized independently and was confirmed by 1H-NMR and HRMS (Fig. S4†). And, the HPLC retention time of compound 1 is the same as the reduction product of Lyso-AFP (Fig. 4), which indicates that 1 is responsible for the fluorescence enhancement at 535 nm.
Generally, realizing higher selectivity toward a specific analyte over other potential competing species is necessary for a fluorescence chemosensor. So, we explored the fluorescence spectral changes of Lyso-AFP (10 μM) incubated with various cations, anions and sulfur-containing analytes in aqueous solutions (CH3CN–HEPES = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, pH = 7.4, Fig. 5). By comparison, when Lyso-AFP was treated with 20 equiv. NaHS, a great fluorescent enhancement was observed. While, the addition of 20 equiv. of Na+, K+, Mg2+, Ca2+, Ag+, Zn2+, F−, Cl−, Br−, ClO4−, HCO3−, NO3−, NO2−, PO43−, HPO42−, H2PO4−, P2O74−, S2O32−, S2O42−, S2O52−, S2O82−, SO3−, N3−, SCN−, CO32−, CH3COO−, SO42−, HSO4−, citrate, hydrogen citrate, dihydrogen citrate, ascorbic acid, L-cysteine, homocysteine, L-glutathione and N-acetyl-L-cysteine exerted a negligible change on the fluorescence response for Lyso-AFP. In this regard, Lyso-AFP can be considered as a good off–on chemosensor for specific recognition of H2S.
50, pH = 7.4, Fig. 5). By comparison, when Lyso-AFP was treated with 20 equiv. NaHS, a great fluorescent enhancement was observed. While, the addition of 20 equiv. of Na+, K+, Mg2+, Ca2+, Ag+, Zn2+, F−, Cl−, Br−, ClO4−, HCO3−, NO3−, NO2−, PO43−, HPO42−, H2PO4−, P2O74−, S2O32−, S2O42−, S2O52−, S2O82−, SO3−, N3−, SCN−, CO32−, CH3COO−, SO42−, HSO4−, citrate, hydrogen citrate, dihydrogen citrate, ascorbic acid, L-cysteine, homocysteine, L-glutathione and N-acetyl-L-cysteine exerted a negligible change on the fluorescence response for Lyso-AFP. In this regard, Lyso-AFP can be considered as a good off–on chemosensor for specific recognition of H2S.
Imaging of lysosomal H2S with Lyso-APF
We next sought to apply Lyso-AFP to the detection of H2S in Hela cells. When incubated with 5 μM Lyso-AFP for 30 min, the cells were washed with phosphate buffered saline (PBS) (pH 7.4) to remove excess of Lyso-AFP. Then, Hela cells exhibited no fluorescence seen from the confocal image (Fig. 6a). While after incubated with 50 μM NaHS for 4 min, the cells displayed enhanced green fluorescence (Fig. 6b). Another 4 min later, a higher turn-on fluorescence response was observed (Fig. 6c). Moreover, the fluorescence intensity reached the maximum in 20 min. All these experiments demonstrated the potential biological application of Lyso-AFP for imaging H2S in living cells.In order to confirm whether Lyso-AFP can specifically stain the lysosomes, Neutral Red (2 μM), a commercially available probe for lysosome, was used to stain the Hela cells at the same time. The yellow parts in Fig. 7c represent the colocalization 1 and NR. The fluorescence patterns of 1 and NR signals merged very well, which indicated the fluorescence response of Lyso-AFP to H2S was mainly located in the lysosomes. The intensity profiles of the linear regions of interest across Hela cells stained with Lyso-AFP and NR also displayed in close synchrony (Fig. 7e). The high Pearson's coefficient and overlap coefficient are 0.970 and 0.971, respectively (Fig. 7f). The cytotoxicity of Lyso-AFP was examined toward Hela cells by a MTT assay (Fig. S1†). The results showed that >90% Hela cells survived after 24 h (5.0 μM Lyso-AFP incubation), demonstrating that Lyso-AFP was of low toxicity toward cultured cell lines.
Conclusion
In summary, we reported a novel fluorescence probe based on 1,8-naphthalimide derivatives which can be used for imaging H2S in lysosomes. The rapid reduction of azide to amine makes Lyso-AFP convert to compound 1 with strong green fluorescence in minutes. Compared with previous work, the probe has better biocompatibility due to its low toxicity, safer byproduct, and insensitivity to pH over lysosomal pH range. Besides, Lyso-AFP was proved to be highly selective for H2S and it did not response to other biological mercaptan. Its potential application in living cells encourages us to actively pursue much more biocompatible fluorescent probes for imaging H2S in different organelles.Acknowledgements
We thank financial supports from the National Natural Science Foundation of China (21276251), Ministry of Human Resources and Social Security of PRC, the 100 talents program funded by Chinese Academy of Sciences, and State Key Laboratory of Fine Chemicals of China (KF1105).Notes and references
- Z. Xu, J. Yoon and D. R. Spring, Chem. Soc. Rev., 2010, 39, 1996–2006 RSC.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef CAS PubMed.
- E. Tomat, E. M. Nolan, J. Jaworski and S. J. Lippard, J. Am. Chem. Soc., 2008, 130, 15776–15777 CrossRef CAS PubMed.
- G. Masanta, C. S. Lim, H. J. Kim, J. H. Han, H. M. Kim and B. R. Cho, J. Am. Chem. Soc., 2011, 133, 5698–5700 CrossRef CAS PubMed.
- Y. Qin, P. J. Dittmer, J. G. Park, K. B. Jansen and A. E. Palmer, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 7351–7356 CrossRef CAS PubMed.
- H. Appelqvist, P. Wäster, K. Kågedal and K. Öllinger, J. Mol. Cell Biol., 2013, 5, 214–226 CrossRef CAS PubMed.
- C. Settembre, A. Fraldi, D. L. Medina and A. Ballabio, Nat. Rev. Mol. Cell Biol., 2013, 14, 283–296 CrossRef CAS PubMed.
- X. Wang, D. M. Nguyen, C. O. Yanez, L. Rodriguez, H.-Y. Ahn, M. V. Bondar and K. D. Belfield, J. Am. Chem. Soc., 2010, 132, 12237–12239 CrossRef CAS PubMed.
- K. Glunde, C. A. Foss, T. Takagi, F. Wildes and Z. M. Bhujwalla, Bioconjugate Chem., 2005, 16, 843–851 CrossRef CAS PubMed.
- Z. Wu, M. Tang, T. Tian, J. Wu, Y. Deng, X. Dong, Z. Tan, X. Weng, Z. Liu, C. Wang and X. Zhou, Talanta, 2011, 87, 216–221 CrossRef CAS PubMed.
- Z. Li, Y. Song, Y. Yang, L. Yang, X. Huang, J. Han and S. Han, Chem. Sci., 2012, 3, 2941–2948 RSC.
- S.-H. Shim, C. Xia, G. Zhong, H. P. Babcock, J. C. Vaughan, B. Huang, X. Wang, C. Xu, G.-Q. Bi and X. Zhuang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 13978–13983 CrossRef CAS PubMed.
- W. Yang, P. S. Chan, M. S. Chan, K. F. Li, P. K. Lo, N. K. Mak, K. W. Cheah and M. S. Wong, Chem. Commun., 2013, 49, 3428–3430 RSC.
- K. Zhou, Y. Wang, X. Huang, K. Luby-Phelps, B. D. Sumer and J. Gao, Angew. Chem., Int. Ed., 2011, 50, 6109–6114 CrossRef CAS PubMed.
- L. Yin, C. He, C. Huang, W. Zhu, X. Wang, Y. Xu and X. Qian, Chem. Commun., 2012, 48, 4486–4488 RSC.
- Y. Urano, D. Asanuma, Y. Hama, Y. Koyama, T. Barrett, M. Kamiya, T. Nagano, T. Watanabe, A. Hasegawa, P. L. Choyke and H. Kobayashi, Nat. Med., 2009, 15, 104–109 CrossRef CAS PubMed.
- K. A. Christensen, J. T. Myers and J. A. Swanson, J. Cell Sci., 2002, 115, 599–607 CAS.
- H. Zhu, J. Fan, S. Zhang, J. Cao, K. Song, D. Ge, H. Dong, J. Wang and X. Peng, Biomater. Sci., 2014, 2, 89–97 RSC.
- F. R. Muylle, D. Adriaensen, W. Coen, J.-P. Timmermans and R. Blust, BioMetals, 2006, 19, 437–450 CrossRef CAS PubMed.
- L. Xue, G. Li, D. Zhu, Q. Liu and H. Jiang, Inorg. Chem., 2012, 51, 10842–10849 CrossRef CAS PubMed.
- K. A. Price, J. L. Hickey, Z. Xiao, A. G. Wedd, S. A. James, J. R. Liddell, P. J. Crouch, A. R. White and P. S. Donnelly, Chem. Sci., 2012, 3, 2748–2759 RSC.
- P. Li, H. Zhou and B. Tang, J. Photochem. Photobiol., A, 2012, 249, 36–40 CrossRef CAS PubMed.
- H. Yu, Y. Xiao and L. Jin, J. Am. Chem. Soc., 2012, 134, 17486–17489 CrossRef CAS PubMed.
- D. Song, J. M. Lim, S. Cho, S.-J. Park, J. Cho, D. Kang, S. G. Rhee, Y. You and W. Nam, Chem. Commun., 2012, 48, 5449–5451 RSC.
- J. Lee and M. Bogyo, ACS Chem. Biol., 2009, 5, 233–243 CrossRef PubMed.
- L. Wang, Y. Xiao, W. Tian and L. Deng, J. Am. Chem. Soc., 2013, 135, 2903–2906 CrossRef CAS PubMed.
- A. Abe, P. W. Rzepecki and J. A. Shayman, Anal. Biochem., 2013, 434, 78–83 CrossRef CAS PubMed.
- M.-J. Wang, W.-J. Cai and Y.-C. Zhu, Clin. Exp. Pharmacol. Physiol., 2010, 37, 764–771 CrossRef CAS PubMed.
- L.-J. Zhang, B.-B. Tao, M.-J. Wang, H.-M. Jin and Y.-C. Zhu, PLoS One, 2012, 7, e44590 CAS.
- K. Eto, T. Asada, K. Arima, T. Makifuchi and H. Kimura, Biochem. Biophys. Res. Commun., 2002, 293, 1485–1488 CrossRef CAS.
- P. Kamoun, M.-C. Belardinelli, A. Chabli, K. Lallouchi and B. Chadefaux-Vekemans, Am. J. Med. Genet., Part A, 2003, 116A, 310–311 CrossRef PubMed.
- W. Yang, G. Yang, X. Jia, L. Wu and R. Wang, J. Physiol., 2005, 569, 519–531 CrossRef CAS PubMed.
- S. Fiorucci, E. Antonelli, A. Mencarelli, S. Orlandi, B. Renga, G. Rizzo, E. Distrutti, V. Shah and A. Morelli, Hepatology, 2005, 42, 539–548 CrossRef CAS PubMed.
- N. S. Cheung, Z. F. Peng, M. J. Chen, P. K. Moore and M. Whiteman, Neuropharmacology, 2007, 53, 505–514 CrossRef CAS PubMed.
- T. Liu, Z. Xu, D. R. Spring and J. Cui, Org. Lett., 2013, 15, 2310–2313 CrossRef CAS PubMed.
- A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078–10080 CrossRef CAS PubMed.
- H. Peng, Y. Cheng, C. Dai, A. L. King, B. L. Predmore, D. J. Lefer and B. Wang, Angew. Chem., Int. Ed., 2011, 50, 9672–9675 CrossRef CAS PubMed.
- S. K. Das, C. S. Lim, S. Y. Yang, J. H. Han and B. R. Cho, Chem. Commun., 2012, 48, 8395–8397 RSC.
- L. A. Montoya and M. D. Pluth, Chem. Commun., 2012, 48, 4767–4769 RSC.
- Q. Wan, Y. Song, Z. Li, X. Gao and H. Ma, Chem. Commun., 2013, 49, 502–504 RSC.
- Z. Wu, Z. Li, L. Yang, J. Han and S. Han, Chem. Commun., 2012, 48, 10120–10122 RSC.
- F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 2852–2854 RSC.
- S. Chen, Z.-j. Chen, W. Ren and H.-w. Ai, J. Am. Chem. Soc., 2012, 134, 9589–9592 CrossRef CAS PubMed.
- T. Nishi and M. Forgac, Nat. Rev. Mol. Cell Biol., 2002, 3, 94–103 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03725a |
| This journal is © The Royal Society of Chemistry 2014 |