Hydrogen-bonding strategy for constructing pH-sensitive core–shell micelles with hydrophilic polymer as the shell and hydrophobic drug as the core†
Wei Wang,
Hong Yang,
Xiangqi Kong,
Zhao Ye,
Yihua Yin*,
Xueqiong Zhang,
Guanghua He,
Peihu Xu and
Hua Zheng*
Department of Pharmaceutical Engineering, School of Chemical Engineering, Wuhan University of Technology, Wuhan, 430070, China. E-mail: yihuayin@yahoo.com.cn
First published on 17th June 2014
Abstract
A hydrogen-bonding assembly strategy for constructing pH-sensitive core–shell micelles with hydrophilic polymer as the shell and hydrophobic drug as the core was described which can achieve the integration of self-assembly and drug-loading. The in vitro release profiles of the micelles show a pH-triggered release.
During past decades, polymeric micelles have attracted much attention because of their high potential for biomedical and pharmaceutical applications.1–3 To sum up, their preparation methods can be divided into three categories based on the structures and the compositions of self-assembled polymer precursors: (1) the self-assembly of block or graft copolymers in selective solvents;4,5 (2) that of interpolymer complexes in selective solvents;6 (3) that of amphiphilic macromolecular conjugates in aqueous solutions.7 Among those methods, for the micelles based on interpolymer complexes, their cores and shells are linked by ionic interactions or hydrogen bonding rather than covalent bonds.5 It has been reported8 that end-functionalized oligomers as the grafts can interact with flexible polymers as a backbone in their common solvent via reversible hydrogen bonding to produce graft-like complexes, and the complexes can assemble into core–shell micelles in a selective solvent. This result stimulated our interests to explore a novel noncovalently connected micelle with hydrophilic polymer as the shell and hydrophobic drug as the core.
We assume that, if a graft-like complex can directly be formed by hydrogen-bond linkage between hydrophobic small-molecule drug and hydrophilic polymer, the complex should be able to self-assemble into a core–shell micelle-like system with drug as the core and polymer as the shell. In the meantime, in consideration of the differential properties between the plasma and some cytoplasmic compartments, if the linkage is chosen which is relatively stable at neutral pH (bloodstream pH 7.3–7.5 and normal cell 7.35), while becomes susceptible to cleavage once the micelle-like system has entered the lower pH environment (endosome pH 5.0–6.5 and lysosome pH 4.5–5.0) inside a cancerous cell,9,10 the selective release of drug can be achieved.
Based on the above concept, a clinically important anticancer drug, melphalan (Mel), was determined: first, the drug has good hydrophobility, in whose structure the carboxylic group and amino group can form hydrogen bonding linkage with a receptor group in linear hydrophilic polymer chain. Second, the drug has a low bioavailability and relatively short plasma half-life because of poor water solubility and easily metabolism, by whose micellization the two defects are expected to be solved.
From the chemical structure, Mel is an amphoteric electrolyte. Its carboxylic group is in a completely ionized state in physiological environment owing to physiological pH (7.4) above its isoelectric-point (4.7),11 while its amine group exists in a free state. Therefore, such a hydrophilic macromolecular compound should be chosen or designed: on the one hand the compound can form stable hydrogen bonding with the amine groups of multiple melphalan molecules at pH 7.4; on the other hand these hydrogen bonds can break in respond to pH stimuli inside target cells. Carboxymethyl chitosan (CMCS) as an important water-soluble polysaccharide was selected for the design because of its good biocompatibility, biodegradability and low toxicity.
In this paper, a hydrophilic carboxymethyl chitosan derivative (Py-CMCS) was prepared through the Schiff base reaction between CMCS and 4-pyridinealdehyde (Py) at room temperature, which can form an “amphiphilic graft-like” complex (Py-CMCS·Mel) with hydrophobic drug melphalan (Mel) by hydrogen bonding interaction. The complex in aqueous solution can self-assemble into a pH-sensitive core–shell micelle with Mel as the core and Py-CMCS as the shell for drug delivery in cancer cells.
Py-CMCS was synthesized as follow: firstly, CMCS was prepared by the carboxymethylation of CS with chloroacetic acid, and then its water solution was mixed with 4-Py ethanol solution at room temperature to obtain Py-CMCS (see Scheme 1 process (i)). The structure of Py-CMCS was characterized by FT-IR and 1H NMR (see Fig. S1 and S2 in the ESI†). Py-CMCS samples with various degrees of substitution of Py were named and are shown in Table 1. Their degrees of substitution to the C-2 amino groups were estimated by an UV analyzer (see Table 1).
 | ||
| Scheme 1 (i) Synthesis of Py-CMCS, (ii) hydrogen-bonding-assisted self-assembly of Py-CMCS and Mel and (iii) formation of hollow nanospheres. | ||
| Sample | Degree of substitution | |
|---|---|---|
| Carboxymethyl groups | Pyridine groups | |
| Py1 | 0.74 | 0.14 |
| Py2 | 0.74 | 0.27 |
| Py3 | 0.74 | 0.43 |
The obtained Py-CMCS is an amphoteric polyelectrolyte containing both carboxyl groups and Py groups. The ζ-potential measurements showed that the compound has an isoelectric point region of 4.8–5.2, where it can flocculate due to the neutralization of the positive and negative charges. In addition, the poorer hydrophobility of Mel in acid media is also unfavourable to the formation of an amphiphilic complex from the combination of the drug and Py-CMCS, so the medium with pH 7.4 was chosen for study of the complexation. In view of the fact that the isoelectric-point of Mel (4.7) just falls in the isoelectric point region of Py-CMCS, it may be sure that the carboxyl groups of Py-CMCS and Mel at pH 7.4 are almost in a completely ionized state. Furthermore, due to the electrostatic repulsion between the negatively charged carboxyl group ions, Mel links the pyridinic moiety of Py-CMCS only with its free amino group by hydrogen bonding (see Scheme 1). This had been confirmed by FT-IR and DSC of Py-CMCS·Mel complex at 7.4 (see Fig. 1). In Fig. 1, the Py characteristic absorption band from a Py-CMCS·Mel complex at 991 cm−1 in curve b has shifted in comparison with the spectrum of Py-CMCS in curve a and the other two characteristic absorption bands have overlapped with the bands at 1560 cm−1 and 1388 cm−1, while the three bands from a mixture of Py-CMCS and Mel remain unchanged (see curve c), suggesting the formation of hydrogen bonding between the amine groups from Mels and nitrogen groups from pyridines.12 The DSC profiles (see Fig. S3 in the ESI†) further support this result. Broad endothermic peaks at 70–120 °C correspond to water evaporation. On comparing the DSC curves of Py-CMCS–Mel (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and Py-CMCS·Mel (0.6
1) mixture and Py-CMCS·Mel (0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or Py-CMCS·Mel (1
1) or Py-CMCS·Mel (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) complex, it was found that the endothermic peaks of the two complexes have shifted to higher values with a smaller peak area, suggesting that the complexes formed a close compacted structure with a lower water holding capacity because of the formation of hydrogen bonding between Py-CMCS and Mel.
1) complex, it was found that the endothermic peaks of the two complexes have shifted to higher values with a smaller peak area, suggesting that the complexes formed a close compacted structure with a lower water holding capacity because of the formation of hydrogen bonding between Py-CMCS and Mel.
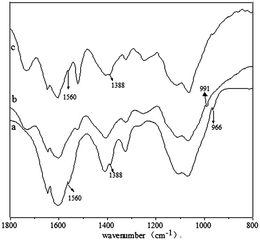 | ||
Fig. 1 FT-IR spectra of Py-CMCS (a), Py-CMCS·Mel micelles-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) obtained at pH 7.4 (b) and Py-CMCS–Mel (1 1) obtained at pH 7.4 (b) and Py-CMCS–Mel (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture (c). 1) mixture (c). | ||
Formation of hydrogen bonds between Mel and Py-CMCS in the medium facilitates phase separation, producing core–shell micelles with Mel in the particle core (see Scheme 1 process (ii)). This was confirmed by dynamic light scattering results (see Fig. S4 in the ESI†). Before addition of Mel, Py-CMCS dissolved in the aqueous solution without phase separation. Addition of Mel induces Py-CMCS phase separation and micelle formation in the solution. The transmission electron microscopy (TEM) image showed in Fig. 2A further supports this result, and the diffraction contrast across each particle suggests that the nanospheres are a core–shell architecture.
To further confirm that the micelles consist of a Mel core and a Py-CMCS shell, the micelles at pH 7.4 were collected through centrifugation. Removal of Mel was conducted using ethanol wash. Ethanol is a good solvent for pyridine. During the ethanol wash, the Py groups in Py-CMCS are solvated selectively by ethanol, which breaks the hydrogen bonding linkage between the Py groups and Mel; the ethanol then dissolves and removes the Mel to form hollow nanospheres (see Scheme 1 process (iii)). The TEM image (see Fig. 2B) shows evident diffraction contrast between the cores and shells of the particles after removal of Mel by ethanol wash, which confirms the formation of hollow nanospheres and also indicates that Mel associates with Py groups and resides at the nanoparticle core surrounded by Py-CMCS. By careful control of the molar ratio of Mel to Py-CMCS, a Mel/Py-CMCS molar ratio range of 0.40–1.12 was determined, above which the micelle diameter (Dh) does not increase and the extra Mel crystallizes from the solution, but below the range, we can control Dh from several nanometers up to more of 144 nanometers by gradually increasing the amount of Mel (see Table 2).
| Py-CMCS·Mel complex (mol ratio) | Dh (nm) | ||
|---|---|---|---|
| Py-CMCS·Mel micelles-1 | Py-CMCS·Mel micelles-2 | Py-CMCS·Mel micelles-3 | |
| a ↓ – sediment.b The micelle samples, Py-CMCS·Mel micelles-1, Py-CMCS·Mel micelles-2 and Py-CMCS·Mel micelles-3, were prepared by Py1, Py2, and Py3, respectively. Each data was derived by measuring the samples prepared in three different batches. The polydispersity index (PDI) values was shown in Table 1 in the ESI. | |||
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
49.2 ± 0.17 | 60.2 ± 0.13 | 66.8 ± 0.16 |
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78.4 ± 0.18 | 97.1 ± 0.16 | 90.1 ± 0.19 |
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90.7 ± 0.20 | 108.5 ± 0.15 | ↓a |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
104.1 ± 0.14 | 120.3 ± 0.19 | ↓a |
1.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
117.5 ± 0.15 | 144.6 ± 0.18 | ↓a |
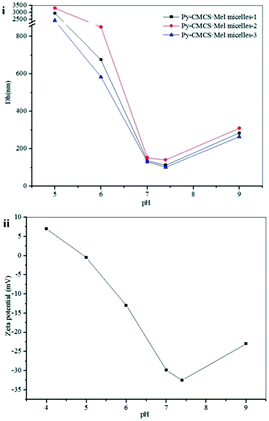
The size of Py-CMCS·Mel micelles can be tuned by changing the pH of solution. A pH range of 5.0 to 9.0, in which Py-CMCS can remain stable (see Fig. S5 in the ESI†), was chosen for study of this responsiveness. As shown in Fig. 3A, each of three samples has a maximum diameter at pH 5.0, and their particle sizes decrease with increasing pH in the range of 5.0–7.4 until reaching a minimum value at pH 7.4, and subsequently increase to some extent again from pH 7.4 to pH 9.0. The change may be ascribed to the charge change on the surface of the micelle shells. As described earlier, Py-CMCS is an amphoteric weak polyelectrolyte with an isoelectric point region of 4.8–5.2. When the pH of the solution falls in the region, the negative and positive charges which Py-CMCS carries are just equal, the micelles tend to form larger aggregates due to decreased electrostatic repulsion, thus the largest diameter and flocculation were observed. However, when the pH is above the region, the negative charges which Py-CMCS carries increase with an increase in pH of the medium (see Fig. 3B). The micelles tend to form smaller particles due to increased electrostatic repulsion. In the experiment, a minimum value was observed at pH 7.4 for the diameter of micelles. The particles had a zeta potential of −32.5 mV, which showed a high stability. Subsequently, a slight increase of the particle diameters from pH 7.4 to pH 9.0 for the three samples may be attributed to the screening of the charges. Such pH-responsive behavior of the Py-CMCS·Mel micelles could be useful for pH-triggered drug delivery.
The in vitro release behavior of Py-CMCS·Mel micelles at three different pH values (pH 5.0, 7.0 and 7.4) are representatively shown in Fig. 4. At pH 5.0, a typical two-phase-release profile was observed. That is, a relatively rapid release in the initial 5 hours followed by a sustained and slow release over a prolonged time up to a maximum cumulative release of 65.1% at 21 hour. By comparison, at pH 7.4 and 7.0 the micelles showed almost the same cumulative release profile. In the initial 20 hours only less than 2% Mel-release was observed and the release reached a plateau phase of 10.04% and 8.81% in the following two hours, respectively. The difference should obviously be ascribed to the hydrogen-bonding interaction between Py-CMCS and Mel in Py-CMCS·Mel micelles. At pH 5.0 the ionization of pyridinic ring groups in Py-CMCS, which destroys the hydrogen-bonding linkage between Py-CMCS and Mel, leads to a faster release with high release percentage; whereas at pH 7.4 and pH 7.0 the linkage is so stable that the drug is prevented from release. The disappearance of hydrogen-bonds at pH 5.0 was detected by FT-IR and the Py characteristic absorption bands from the flocculate at 996 cm−1, 1388 cm−1 and 1560 cm−1 are shown again (see Fig. S6 in the ESI†). This pH-dependent release behavior is of particular interest in achieving the tumor-targeted Mel delivery with micelles. It is expected that most Mel encapsulated in micelles can remain in their interior for a considerable time during systemic circulation (pH 7.4). However, a rapid release will occur once the micelles reach the interior of tumor cells by endocytosis where pH values are lower than those in the normal cells.13
 | ||
Fig. 4 In vitro release profiles for Py-CMCS·Mel-2 (1.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) micelles at pH 5.0, 7.0 and 7.4 in PBS (37 °C). 1) micelles at pH 5.0, 7.0 and 7.4 in PBS (37 °C). | ||
To demonstrate the feasibility of the self-assembled polymer-drug micelles for efficient delivery of anti-cancer drug into tumor cells, a human breast cancer cell line SKBR3 was selected as a model for in vitro cytotoxicity study. The SKBR3 cells were incubated with either free Mel or Py-CMCS or Py-CMCS·Mel micelles. Their inhibition against the cells was evaluated by the MTT assay. It was found in Fig. 5 that blank Py-CMCS showed no cytotoxicity in the concentration lower than 20 μg ml−1, while the Py-CMCS·Mel micelles exhibited comparable cytotoxic effect to free Mel in the drug concentration range of 0.39–0.68 μM. The Mel doses required for 50% cellular growth inhibition (IC50) were measured as 0.15 μg ml−1 and 0.12 μg ml−1 for Py-CMCS·Mel micelles and free Mel, respectively. These data indicated that the Py-CMCS·Mel micelles were efficiently taken up by the SKBR3 cells and released Mel in an acidic intracellular milieu. Because free Mel can harm normal cells before reaching target cells, whose practical therapeutic efficiency is limited unless increasing its concentration, which can lead to more serious side-effects. The Py-CMCS·Mel micelles, however, will have a very low cytotoxicity to normal cells because of the lower Mel leakage (<10 wt% within twenty hours), while can rapidly release drug via a pH-triggered mechanism upon entering cancer cells, thus significantly enhancing the therapeutic efficiency of Mel to tumor cells.
 | ||
| Fig. 5 MTT assay of in vitro cytotoxicity of (i) free Mel and Py-CMCS·Mel micelles; (ii) Py-CMCS against SKBR3 cells (each bars represent means ± SD for n = 3, p < 0.05). | ||
In summary, we have demonstrated a hydrogen-bonding strategy for constructing pH-sensitive micelles with hydrophilic polymer as the shell and hydrophobic drug as the core. Compared with the traditional method of preparing drug-loaded micelles, this approach can achieve the integration of self-assembly and drug-loading of micelles-based drug delivery system, avoiding the multiple steps that currently used in the preparation of drug-loaded micelles. In addition, the system constructed by the approach can undergo dynamic changes in structure in response to environmental stimuli (pH value) to achieve the controlled release of anti-cancer drug in targeting cells. Furthermore, the carboxylic groups located at the surface of the micelles can be available as a conjugation site of various targeting ligands. Finally, it may also be possible to develop this approach to other system based on weak interactions between polymers and drug or other small molecules, such as electrostatic and hydrogen bonds interaction.
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (nos 51373130 and 51273156), the Natural Science Foundation for the Youth (no. 51303145) and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (no. 20131049720005).Notes and references
- Y. L. Colson and M. W. Grinstaff, Adv. Mater., 2012, 24, 3878–3886 CrossRef CAS.
- F. E. Alemdaroglu, N. C Alemdaroglu, P. Langguth and A. Herrmann, Adv. Mater., 2008, 20, 899–902 CrossRef CAS.
- L. Tang, V. Tjong, N. Li, Y. G. Yingling, A. Chilkoti and S. Zauscher, Adv. Mater., 2014, 1–5 Search PubMed.
- H. G. Cui, Z. Y. Chen, S. Zhong, K. L. Wooley and D. J. Pochan, Science, 2007, 317, 647–650 CrossRef CAS PubMed.
- S. Y. Liu, M. Jiang, H. J. Liang and C. Wu, Polymer, 2000, 41, 8697–8702 CrossRef CAS.
- X. K. Liu and M. Jiang, Angew. Chem., 2006, 118, 3930–3934 CrossRef.
- Y. Wang, H. Xu and X. Zhang, Adv. Mater., 2009, 21, 2849–2864 CrossRef CAS.
- C. H. Hsu, S. W. Kuo, J. K. Chen, F. H. Ko, C. S. Liao and F. C. Chang, Langmuir, 2008, 24, 7727–7734 CrossRef CAS PubMed.
- J. Z. Du, T. M. Sun, W. J. Song, J. Wu and J. Wang, Angew. Chem., Int. Ed., 2010, 49, 1–7 CrossRef PubMed.
- Y. Bae, S. Fukushima, A. Harada and K. Kataoka, Angew. Chem., Int. Ed, 2003, 42, 4640–4643 CrossRef CAS PubMed.
- S. A. Stout and C. M. Riley, Int. J. Pharm., 1985, 24, 193–208 CrossRef CAS.
- C. D. Liang, K. L. Hong, G. A. Guiochon, J. W. Mays and S. Dai, Angew. Chem., Int. Ed, 2004, 43, 5785–5789 CrossRef CAS PubMed.
- X. T. Shuai, H. Ai, N. Nasongkla, S. Kim and J. M. Gao, J. Controlled Release, 2004, 98, 415–426 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03716b |
| This journal is © The Royal Society of Chemistry 2014 |