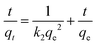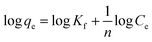DOI:
10.1039/C4RA03692A
(Paper)
RSC Adv., 2014,
4, 42376-42382
Fabrication of α-Fe2O3–γ-Al2O3 core–shell nanofibers and their Cr(VI) adsorptive properties†
Received
23rd April 2014
, Accepted 1st September 2014
First published on 3rd September 2014
Abstract
α-Fe2O3–γ-Al2O3 core–shell nanofibers have been synthesized via an electrospinning process combined with vapor deposition and heat treatment techniques. The composite nanofibers exhibited ferromagnetic properties and Cr(VI) removal performance. The Freundlich adsorption isotherm was applied to describe the adsorption process. Kinetics of the Cr(VI) ion adsorption were found to follow a pseudo-second-order rate equation. The obtained α-Fe2O3–γ-Al2O3 core–shell nanofibers were carefully examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and vibrating sample magnetometry (VSM). The adsorption mechanism for Cr(VI) onto α-Fe2O3–γ-Al2O3 core–shell nanofibers was elucidated by X-ray photoelectron spectroscopy (XPS). The results suggested that the electrostatic adsorption between the positively charged surface of α-Fe2O3–γ-Al2O3 nanofibers and Cr(VI) species, and the electron–hole pair provided by Fe2O3 induced the Cr(VI) reduction to Cr(III). It is anticipated that the α-Fe2O3–γ-Al2O3 core–shell nanofibers are an attractive adsorbent for the removal of heavy metal ions from water.
Introduction
The increasing worldwide contamination of freshwater systems has become one of the key environmental problems facing humanity. Heavy metal ion pollution is regarded as the most severe environmental contamination today. Various kinds of methods have been carried out for heavy metal ion removal such as precipitation, membrane filtration, reverse osmosis, solvent extraction and electrolysis, biological treatment, ion-exchange processes, and adsorption. Among them, adsorption technique has advantage of high efficiency, cost effectiveness and eco-friendly materials as adsorbents.1,2
Iron or aluminum oxides and hydroxides are well known to show high uptake of cations and anions3 in natural environments, such as chromium,4 arsenate5 and phosphate.6 α-Fe2O3 and γ-Al2O3 are more suitable for the removal of toxic heavy metal ions and organic pollutants from wastewater because of their high thermal stabilities, large surface areas, low cost and abundant availability.7,8 Recently, Chubar et al. prepared Fe2O3·Al2O3·xH2O with high specific surface area by a sol–gel method, and the Fe2O3·Al2O3·xH2O adsorbent showed good adsorption ability for phosphate ions.9 Gulshan et al. synthesized alumina–iron oxide compounds by a gel evaporation method and investigated its simultaneous uptake properties for Ni2+, NH4+ and H2PO4−.10 Li et al. prepared three types of alumina-coupled iron oxides by coprecipitation approach and investigated their photocatalytic activity for bisphenol A degradation.11 Cao et al. synthesized flowerlike α-Fe2O3 nanostructures with maximum capacities of 51 and 30 mg g−1 for As(V) and Cr(VI) removal.12 Wei et al. obtained hollow nestlike α-Fe2O3 nanostructures with maximum removal capacities of 75.3, 58.5, and 160 mg g−1 for As(V), Cr(VI), and Congo red.13 Ge et al. prepared the hierarchical γ-Al2O3 with a facile hydrothermal method and this product exhibited fast and high adsorption capacities towards Cr(VI) and CO2.14 Mahapatra et al. investigated Fe2O3–Al2O3 nanocomposite fibers removal behavior of heavy metal ions from aqueous solution, the removal percentage was in the order of Cu2+< Pb2+< Ni2+< Hg2+.15
Currently, nanomaterials and nanotechnology have garnered worldwide attention for their application in environmental remediation and pollution control, because nanostructured surfaces offer large surface areas and rich valence states that provide enhanced affinity and adsorption capability toward pollutants.15 Electrospinning technique has been known to be a simple and versatile method to generate organic or inorganic nanofibers. This technique involves applying a high voltage on a viscous solution to fabricate ultrathin fibers with diameters in the range of 20–2000 nm. These nanofibers show a number of interesting characteristics such as large surface area per unit mass, high gas permeability, high porosity and small pore size. Therefore in recent studies, electrospun fibers have been widely used as matrixes or templates to fabricate functional materials with tunable structures, such as core–shell nanofibers,16 molecular sieve fibers,17 nanotubes,18 necklace-like nanostructures,19 hierarchically nanofibrous membrane,20 functional-group chelating nanofiber membranes,21 and polymer-supported nanocomposite.22
In this work, we have reported the preparation of a novel fiber-in-tube nanofibers containing of α-Fe2O3 nanofiber core and γ-Al2O3 shell via an electrospinning technique combining with vapor deposition and heat treatment techniques. The α-Fe2O3–γ-Al2O3 core–shell nanofibers exhibited ferromagnetic property and Cr(VI) removal performance. Therefore, this work provides a facile and effective approach for the fabrication of α-Fe2O3–γ-Al2O3 core–shell nanofibers, which can be potentially applied in heavy metal ions removal from wastewater effluents.
Experimental
Materials
Poly(vinyl alcohol) (PVA, Mw = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–80
000–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Beijing Chemical Factory. Triton-X 100 (C34H62O11) was purchased from Aldrich. Ammonium ferric citrate [Fe(NH4)3(C6H5O7)2] was purchased from Shantou Xilong Chemical Corporation. Potassium dichromate (K2Cr2O7) was purchased from Beijing Chemical Factory. All the reagents were used without further purification.
000) was purchased from Beijing Chemical Factory. Triton-X 100 (C34H62O11) was purchased from Aldrich. Ammonium ferric citrate [Fe(NH4)3(C6H5O7)2] was purchased from Shantou Xilong Chemical Corporation. Potassium dichromate (K2Cr2O7) was purchased from Beijing Chemical Factory. All the reagents were used without further purification.
Characterization
The morphology of the nanofibers was characterized by scanning electron microscopy (SEM, Shimadzu SSX-550). Transmission electron microscope (TEM) measurements were conducted on a JEOL JEM 1200EX at 100 kV. HRTEM imaging was performed on a FEI Tecnai G2 F20 high-resolution transmission electron microscope operating at 200 kV. FT-IR spectra were recorded on a Bruker Vector-22 FT-IR spectrometer from 4000 to 400 cm−1 using powder-pressed KBr pellets at room temperature. The amounts of initial Cr(VI) ions were determined using an inductive coupled plasma emission spectrometer (ICP, PerkinElmer OPTIMA 3300DV). The pH values of the reaction solutions were measured by using a pH meter (STARTER 2100). The ultraviolet-visible (UV-vis) absorption spectroscopy of Cr(VI) ions solution were recorded using SHIMADZU UV-2501 UV-vis spectrophotometer. Analysis of the X-ray photoelectron spectra (XPS) was performed on Thermo ESCALAB 250 spectrometer with a Mg–K (1253.6 eV) achromatic X-ray source. The static magnetic properties of the α-Fe2O3–γ-Al2O3 core–shell nanofibers were measured using a vibrating sample magnetometer (VSM 7410) at a temperature of 300 K.
Electrospinning of ammonium ferric citrate/PVA composite nanofibers
PVA aqueous solution was prepared by dissolving 0.65 g of PVA powder in 9.35 g of deionized water, heating to 80 °C under stirring for 2 h, and then cooling down to room temperature. Afterward, 0.385 g of ammonium ferric citrate was added into the PVA aqueous solution and stirred for 3 h. Then the solution was loaded into a glass syringe and connected to high-voltage power supply (a Gamma High Voltage Research ORMOND BEACH.FL32174 dc power supply). 18 kV was provided between the cathode and anode at a distance of 15 cm to prepare ammonium ferric citrate/PVA composite nanofibers, and several silicon slices (size: 3 cm × 3 cm) were used as the collector, which was sticked on the surface of Al foil.
Preparation of α-Fe2O3–γ-Al2O3 core–shell nanofibers
Preparation of the aluminum phase. The Al vapor deposition on the ammonium ferric citrate/PVA composite nanofibers was performed in a commercial thermal evaporation system at a pressure of 5 × 10−4 Pa (KYKY Technology Development Co. Ltd. China). The depositing rate was 20 Å s−1 and the thickness of the Al film was 150–190 nm. The ammonium ferric citrate/PVA–Al composite nanofibers were calcined in air with programmable heating process. The heating rate is 10 °C min−1, the samples first were annealed at 600 °C for 2 h, and then heating up to 800 °C for 2 h. After the fibers were naturally cooled to room temperature, the α-Fe2O3–γ-Al2O3 core–shell nanofibers were obtained.
Adsorption of Cr(VI) ions
The Cr(VI) solution was prepared by dissolving potassium dichromate (K2Cr2O7) in aqueous solution at pH = 2.0. The adsorption isotherm of Cr(VI) ions on α-Fe2O3–γ-Al2O3 core–shell nanofibers was studied by dispersing the samples (2 mg) in a series of flasks containing 45.0 mL Cr(VI) ions solution of varying initial concentrations, ranging from 9.0 to 62.0 mg L−1. The flasks were placed on a shaker and kept at room temperature. After shaking for a certain time the solution was separated for concentration analysis, the initial concentration of the Cr(VI) ions in solution was determined by ICP spectrometer. The equilibrium concentration of Cr(VI) ions in the solution was measured by UV-vis absorption spectroscopy. The amount of the Cr(VI) ions adsorbed onto each sample (the adsorption capacity (q, in mg g−1)) was calculated on the basis of the following equation:23| |
 | (1) |
where C0 and Ce are the initial and the equilibrium concentrations of the metal ions in the test solution (mg L−1), V is the volume of the testing solution (L), and W is the weight of the adsorbent (g).
Results and discussion
Morphologies
The morphologies of the as-electrospun ammonium ferric citrate/PVA composite nanofibers and α-Fe2O3–γ-Al2O3 core–shell nanofibers were observed by SEM and TEM measurements. As shown in Fig. 1a, the obtained ammonium ferric citrate/PVA composite nanofibers have a basic smooth and uniform structure, with the diameter in range of 300–650 nm (Fig. 1d). After vapor deposition and annealing, the composite nanofibers keep the smooth fiber morphology (Fig. 1b), with an average diameter of ∼330 nm (Fig. 1e). Fig. 1c shows the TEM image of the obtained nanofibers. The core–shell structure can been clearly seen. The core is α-Fe2O3 nanofibers derived from ammonium ferric citrate/PVA composite nanofibers and the shell is γ-Al2O3. After calcination (Fig. 1c and f), it can also be clearly seen that the α-Fe2O3 core fibers have rough surface, and the diameter is in the range of 53–76 nm, due to the crystallization related to the formation of particulate morphology. A HRTEM image taken from the edge of (Fig. 2a) was shown in Fig. 2b. The typical lattice fringes were clearly visible, with a spacing of 0.37 and 0.27 nm, corresponding to the (012) and (104) planes of α-Fe2O3, respectively. The spacing of 0.20 nm was corresponded to the (400) planes of γ-Al2O3, which further indicates that the nanofibers consisted of α-Fe2O3 core and γ-Al2O3 shell.
 |
| | Fig. 1 SEM images of (a) ammonium ferric citrate/PVA composite nanofibers, (b) α-Fe2O3–γ-Al2O3 core–shell nanofibers. TEM images of (c), (f) α-Fe2O3–γ-Al2O3 core–shell nanofibers. The distribution of fiber diameters from (d) the ammonium ferric citrate/PVA composite nanofibers, and (e) α-Fe2O3–γ-Al2O3 core–shell nanofibers. | |
 |
| | Fig. 2 TEM (a) and HRTEM (b) images of the as-prepared α-Fe2O3–γ-Al2O3 core–shell nanofibers. | |
Structural properties
The chemical composition of the as-prepared product was analyzed using powder XRD measurements. As shown in Fig. 3, the diffraction angles at 2θ = 24.33°, 33.27°, 35.79°, 41.00°, 49.60°, 54.20°, 57.76°, 62.63°, 64.10° and 72.17° can be assigned to (012), (104), (110), (113), (024), (116), (018), (214), (300) and (119) planes of α-Fe2O3, which are in good agreement with the recorded values of JCPDS File Card no. 33-0664. The pronounced peaks at (012), (104), and (110) suggest that the obtained α-Fe2O3 was well-crystallized. The peaks at 38.55°, 46.03° and 67.14° degrees are assigned to the (222), (400) and (440) reflection of γ-Al2O3 (JCPDS File Card no. 10-0425). The results indicate that α-Fe2O3–γ-Al2O3 nanofibers have been successfully prepared.
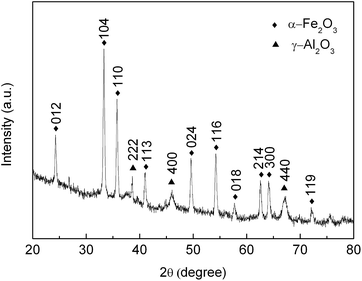 |
| | Fig. 3 XRD patterns of α-Fe2O3–γ-Al2O3 core–shell nanofibers. | |
The FT-IR spectra shown in Fig. 4 were used to get information for the structural changes related to the pure PVA nanofibers, the ammonium ferric citrate/PVA composite nanofibers and the annealed α-Fe2O3–γ-Al2O3 core–shell nanofibers. For pure PVA nanofibers (Fig. 4a), a broad peak at about 3420 cm−1 corresponds to H–OH stretching vibration, the peak at 2950 cm−1 is related to –CH stretching vibrations, the peaks in the range of 1450–1300 cm−1 and 1000–1160 cm−1 could be ascribed to the –OH bending vibrations and C–O stretching vibrations, respectively. For the ammonium ferric citrate/PVA composite nanofibers (Fig. 4b), the peaks at 1400 cm−1 corresponds to the COO– stretching vibrations. As shown in Fig. 4c, these characteristic peaks of PVA were almost removed from the nanofibers after calcination at 800 °C, illustrating the decomposition of organic matter. Furthermore, two peaks around 541 and 463 cm−1 were observed, which can be ascribed to the metal–oxygen vibration of the α-Fe2O3 and γ-Al2O3.24,25
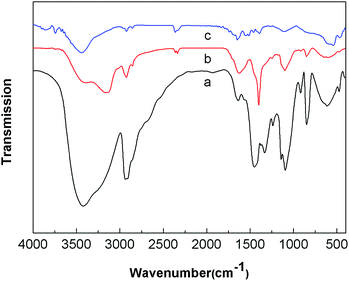 |
| | Fig. 4 FT-IR spectra of (a) PVA nanofibers, (b) the ammonium ferric citrate/PVA composite nanofibers, and (c) α-Fe2O3–γ-Al2O3 core–shell nanofibers. | |
Magnetic behavior
The magnetic behaviors of the samples were conducted by isothermal magnetic hysteresis measurements at room temperature (300 K) with the field sweeping from −20 to 20 kOe. As shown in Fig. 5, the loop suggests that the α-Fe2O3–γ-Al2O3 core–shell nanofibers are ferromagnetic at room temperature, mainly because of the α-Fe2O3 contents display ferromagnetic behavior. The coercivity force (Hc), remnant magnetization (Mr) and saturated magnetization (Ms) of the samples are estimated to be 2.24 kOe, 0.0585 emu g−1 and 0.111 emu g−1, respectively. The observed coercivity of the α-Fe2O3–γ-Al2O3 core–shell nanofibers is larger than that of the rod,26 nanofiber,27 shuttle-like α-Fe2O3,28 Al3+ doped α-Fe2O3 nanoplates,29 and Fe–SiO2 core–shell nanostructures,30 indicating that the magnetization of ferromagnetic materials is sensitive to microstructural characteristics such as size, shape and defects in crystal structure.31
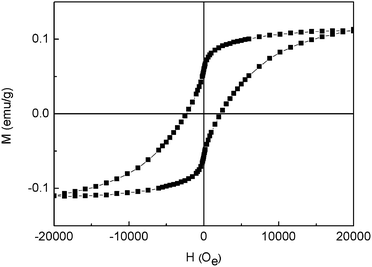 |
| | Fig. 5 Magnetic hysteresis loops of α-Fe2O3–γ-Al2O3 core–shell nanofibers at room temperature. | |
Adsorption kinetics
The core–shell structures of the obtained α-Fe2O3–γ-Al2O3 nanofibers afford its potential applications for waste adsorption. In this study, the feasibility of α-Fe2O3–γ-Al2O3 core–shell nanofibers as Cr(VI) ion remover was explored. Fig. 6 shows the adsorption capacity of Cr(VI) ions in the aqueous solution at different times. Clearly, the adsorption for the Cr(VI) ions capacity increased with the increasing of the contact time, and reached equilibria at about 120 min. Here, we use the pseudo-second-order model to evaluate the kinetics of Cr(VI) removal on α-Fe2O3–γ-Al2O3 core–shell nanofibers (Fig. 7). The linearized form of the pseudo-second-order equation is as follows:21| |
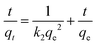 | (2) |
where k2 (g mg−1 min−1) is the pseudo-second-order rate constant, qe (mg g−1) is the adsorption capacity at adsorption equilibrium and qt (mg g−1) is the adsorption at time t. The kinetics of the removal process was shown in Fig. 7. Table 1 summarizes the calculated qe values, pseudo-second-order rate constants k2, and correlation coefficient values (R2). The calculated qe is calculated from the slope and intercept of the plots of t/qt versus t.
 |
| | Fig. 6 The adsorption capacity of α-Fe2O3–γ-Al2O3 core–shell nanofibers for different concentrations of Cr(VI) ions as a function of time. | |
 |
| | Fig. 7 The pseudo-second-order model for adsorption of Cr(VI) ions by α-Fe2O3–γ-Al2O3 core–shell nanofibers. | |
Table 1 Kinetics parameters for Cr(VI) adsorption onto α-Fe2O3–γ-Al2O3 core–shell nanofibers with different concentrations
| Concentration of Cr(VI) ions (mg L−1) |
qe (mg g−1) |
k2 (g mg−1 min−1) |
R2 |
| 10.7 |
13.45 |
0.00314 |
0.9930 |
| 19.7 |
18.82 |
0.00167 |
0.9915 |
| 32.4 |
34.83 |
0.00048 |
0.9646 |
| 41.8 |
39.00 |
0.00054 |
0.9835 |
| 61.7 |
57.34 |
0.00046 |
0.9779 |
The Freundlich isotherm is an empirical equation that is used to describe adsorption at multilayer and adsorption on a heterogeneous surface. It is expressed by the following linear form.20
| |
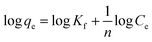 | (3) |
Kf and
n are Freundlich isotherm constants that are related to the adsorption capacity and the adsorption strength of the adsorbent, respectively.
Kf and
n can be obtained from the intercept and the slope of the linear plot of log(
qe)
versus log(
Ce), as shown in
Fig. 8, are summarized in
Table 2. According to the obtained results, the adsorption data of the Cr(
VI) ions on the α-Fe
2O
3–γ-Al
2O
3 core–shell nanofibers are fitted particularly well with the Freundlich model, as indicated by the very high values of the correlation coefficient (
R2 over 0.95). It is usually accepted that a value of 2 ≤
n < 10 represents an easy adsorption, a value of 1 ≤
n < 2 represents a moderate adsorption, while a value of
n < 1 represents a difficult adsorption.
32 For the obtained core–shell structure nanofibers, the value of
n is 1.254. This indicates that the adsorption is not very strong.
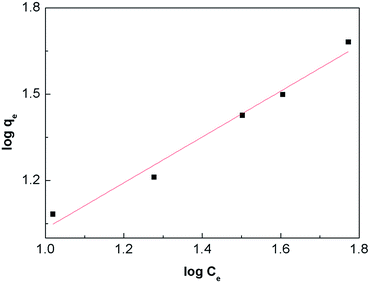 |
| | Fig. 8 Freundlich adsorption isotherm of Cr(VI) onto α-Fe2O3–γ-Al2O3 core–shell nanofibers at room temperature. | |
Table 2 Freundlich parameters for adsorption of Cr(VI) onto α-Fe2O3–γ-Al2O3 core–shell nanofibers
| Metal ion |
Kf |
n |
R2 |
| Cr(VI) |
1.718 |
1.254 |
0.9739 |
The recyclable property of the obtained adsorbent is very important for the materials used in the heavy metal ions removal. We repeated the Cr(VI) removal experiment for three times using the same absorbent after the regeneration. 0.5 M NaOH eluent was used for the desorption process. The adsorption capacity decreases for each new cycle after desorption. In the third cycle the adsorption capacity of the absorbent still remains 81.3% of the first cycle (ESI Fig. S1†).
Adsorption mechanism of Cr(VI) ions with α-Fe2O3–γ-Al2O3 core–shell nanofibers
XPS was further used to study the surface chemical compositions of the as-prepared and Cr(VI) ions adsorbed α-Fe2O3–γ-Al2O3 core–shell nanofibers. Fig. 9 shows the two survey spectra before and after adsorption of Cr(VI) ions. After adsorption, the presence of Cr on the surface of the α-Fe2O3–γ-Al2O3 core–shell nanofibers was obviously observed. High-resolution spectra of Cr 2p (Fig. 9b) could be deconvoluted into two components. The peaks at 576.6 eV (Cr 2p3/2) and 586.3 eV (Cr 2p1/2) are assigned to Cr(III) for Cr2O3. And the other peaks at 579.2 eV (Cr 2p3/2) and 588.5 eV (Cr 2p1/2) are related to Cr(VI) for K2Cr2O7.33 This indicated that the adsorbed Cr on α-Fe2O3–γ-Al2O3 core–shell nanofibers existed two states, implying that the adsorption process involved the some reduction of Cr(VI) to Cr(III). As shown In Fig. 9c, before Cr(VI) adsorption two distinct peaks at binding energies of 710.6 eV for Fe 2p3/2 and 724.2 eV for Fe 2p1/2 with a shake-up satellite at 719.3 eV are observed. This is the characteristic of Fe(III) in Fe2O3.34 Then the binding energy of the Fe 2p shifted to high energy after Cr(VI) adsorption, due to the Fe2O3 as a semiconductor oxide was started by the adsorption of a photon and generating electron–hole pairs during the adsorption process. The peak at 74.7 eV (Al 2p) is assigned to aluminum atoms in Al2O3 (in Fig. 9d).
 |
| | Fig. 9 XPS spectra of α-Fe2O3–γ-Al2O3 core–shell nanofibers before (bottom curve) and after (top curve) adsorption of Cr(VI) ions. | |
In this work, the Cr(VI) is removed through two processes. The first is electrostatic adsorption. The adsorption property of the surface of Fe2O3 and Al2O3 strongly depends on pH in solution. When the pH is low, the Fe2O3 and Al2O3 can be protonated. Its surface is charged positively, and electrostatic attraction between the charged surface and negatively charged HCrO4− and CrO42− in the acid solution (pH = 2).14,20 The second process is redox reaction. The Fe2O3 could produce electron–hole pair under sunlight irradiation, which might be responsible for the Cr(VI) reduction to Cr(III). Under sunlight irradiation and low pH value, electrons and holes were produced and the three-electron reaction of Cr(VI) reduced to Cr(III) could happen.35,36
Conclusions
In this study, the α-Fe2O3–γ-Al2O3 core–shell nanofibers were prepared via an electrospinning technique combining with vapor deposition and heat treatment techniques. The composite nanofibers exhibit ferromagnetic property and Cr(VI) removal performance. Kinetics of the Cr(VI) ions adsorption was found to follow pseudo-second-order rate equation. The adsorption of Cr(VI) ions was fitted well with the Freundlich isotherm equation. The regeneration studies demonstrated that the α-Fe2O3–γ-Al2O3 core–shell nanofibers can be recovered effectively. The adsorption mechanism for Cr(VI) on α-Fe2O3–γ-Al2O3 core–shell nanofibers was confirmed as electrostatic adsorption between hydroxyl on the α-Fe2O3–γ-Al2O3 surface and Cr(VI) species, and the reduction of Cr(VI) to Cr(III). Based on the obtained results, the novel α-Fe2O3–γ-Al2O3 core–shell nanofibers may be applied as a promising adsorbent in water treatment.
Acknowledgements
This work was supported by the research Grants from the National 973 Project (no. S2009061009), the National Key Technology Research and Development Program (2013BAC01B02), the National Natural Science Foundation of China (nos 21274052 and 51303060), Jilin Provincial Science and Technology Department Project (no. 20130206064GX), and Research Fund for the Doctoral Program of Higher Education of China (no. 20120061120017).
Notes and references
- H. Liu and C. Y. Wang, RSC Adv., 2014, 4, 3864–3872 RSC.
- J. M. Gong, T. Liu, X. Q. Wang, X. L. Hu and L. Z. Zhang, Environ. Sci. Technol., 2011, 45, 6181–6187 CrossRef CAS PubMed.
- M. Rovira, J. Giménez, M. Martínez, X. Martínez-Lladó, J. Pablo, V. Martí and L. Duro, J. Hazard. Mater., 2008, 150, 279–284 CrossRef CAS PubMed.
- J. Hu, G. H. Chen and I. M. C. Lo, Water Res., 2005, 39, 4528–4536 CrossRef CAS PubMed.
- T. Basu, K. Gupta and U. C. Ghosh, Chem. Eng. J., 2012, 183, 303–314 CrossRef CAS PubMed.
- A. F. Sousa, T. P. Braga, E. C. C. Gomes, A. Valentini and E. Longhinotti, Chem. Eng. J., 2012, 210, 143–149 CrossRef PubMed.
- S. L. S. Stipp, M. Hansen, R. Kristensen, M. F. Hochella, L. Bennedsen, K. Dideriksen, T. Balic-Zunic, D. Léonard and H.-J. Mathieu, Chem. Geol., 2002, 190, 321–337 CrossRef CAS.
- G. Horányi and P. Joó, J. Colloid Interface Sci., 2002, 247, 12–17 CrossRef PubMed.
- N. I. Chubar, V. A. Kanibolotskyy, V. V. Strelko, G. G. Gallios, V. F. Samanidou, T. O. Shaposhnikova, V. G. Milgrandt and I. Z. Zhuravlev, Colloids Surf., A, 2005, 255, 55–63 CrossRef CAS PubMed.
- F. Gulshan, Y. Kameshima, A. Nakajima and K. Okada, J. Hazard. Mater., 2009, 169, 697–702 CrossRef CAS PubMed.
- F. B. Li, X. Z. Li, C. S. Liu and T. X. Liu, J. Hazard. Mater., 2007, 149, 199–207 CrossRef CAS PubMed.
- C. Y. Cao, J. Qu, W. S. Yan, J. F. Zhu, Z. Y. Wu and W. G. Song, Langmuir, 2012, 28, 4573–4579 CrossRef CAS PubMed.
- Z. H. Wei, R. Xing, X. Zhang, S. Liu, H. H. Yu and P. C. Li, ACS Appl. Mater. Interfaces, 2013, 5, 598–604 CAS.
- J. R. Ge, K. J. Deng, W. Q. Cai, J. G. Yu, X. Q. Liu and J. B. Zhou, J. Colloid Interface Sci., 2013, 401, 34–39 CrossRef CAS PubMed.
- A. Mahapatra, B. G. Mishra and G. Hota, J. Hazard. Mater., 2013, 258–259, 116–123 CrossRef CAS PubMed.
- Z. C. Sun, E. Zussman, A. L. Yarin and J. H. Wendorff, Adv. Mater., 2003, 15, 1929–1932 CrossRef CAS PubMed.
- J. C. Di, Y. Zhao and J. H. Yu, J. Mater. Chem., 2011, 21, 8511–8520 RSC.
- S. K. Li, X. F. Lu, X. Li, Y. P. Xue, C. C. Zhang, J. Y. Lei and C. Wang, J. Colloid Interface Sci., 2012, 378, 30–35 CrossRef CAS PubMed.
- Y. Jin, D. Y. Yang, D. Y. Kang and X. Y. Jiang, Langmuir, 2010, 26, 1186–1190 CrossRef CAS PubMed.
- G. R. Xu, J. N. Wang and C. J. Li, Chem. Eng. J., 2012, 198–199, 310–317 CrossRef CAS PubMed.
- X. Li, C. C. Zhang, R. Zhao, X. F. Lu, X. R. Xu, X. T. Jia, C. Wang and L. J. Li, Chem. Eng. J., 2013, 229, 420–428 CrossRef CAS PubMed.
- Y. Lin, W. Cai, X. Tian, X. Liu, G. Wang and C. Liang, J. Mater. Chem., 2011, 21, 991–997 RSC.
- H. L. Lu, H. Xu, Y. Chen, J. L. Zhang and J. X. Zhuang, RSC Adv., 2014, 4, 5873–5879 RSC.
- X. L. Hu and J. C. Yu, Adv. Funct. Mater., 2008, 18, 880–887 CrossRef CAS PubMed.
- Y. N. Jia, D. R. Chen and X. L. Jiao, Chem. Res. Chin. Univ., 2011, 32, 601–604 CAS.
- S. Y. Zeng, K. B. Tang and T. W. Li, J. Colloid Interface Sci., 2007, 312, 513–521 CrossRef CAS PubMed.
- Y. Zhu, J. C. Zhang, J. Zhai and L. Jiang, Thin Solid Films, 2006, 510, 271–274 CrossRef CAS PubMed.
- X. M. Liu, S. Y. Fu, H. M. Xiao and C. J. Huang, J. Solid State Chem., 2005, 178, 2798–2803 CrossRef CAS PubMed.
- R. M. Liu, Y. W. Jiang, Q. Y. Lu, W. Du and F. Gao, CrystEngComm, 2013, 15, 443–446 RSC.
- S. F. Wang, H. M. Cao, F. Gu, C. Z. Li and G. J. Huang, J. Alloys Compd., 2008, 457, 560–564 CrossRef CAS PubMed.
- C. H. Xia, C. G. Hu, Y. F. Xiong and N. Wang, J. Alloys Compd., 2009, 480, 970–973 CrossRef CAS PubMed.
- F. Z. Mou, J. G. Guan, Z. D. Xiao, Z. G. Sun, W. D. Shi and X. A. Fan, J. Mater. Chem., 2011, 21, 5414–5421 RSC.
- D. Park, S. R. Lim, Y. S. Yun and J. M. Park, Bioresour. Technol., 2008, 99, 8810–8818 CrossRef CAS PubMed.
- X. L. Hu, J. C. Yu, J. M. Gong, Q. Li and G. S. Li, Adv. Mater., 2007, 19, 2324–2329 CrossRef CAS PubMed.
- A. Idris, E. Misran and N. M. Yusof, J. Ind. Eng. Chem., 2012, 18, 2151–2156 CrossRef CAS PubMed.
- A. Idris, N. Hassan, N. S. M. Ismail, E. Misran, N. M. Yusof, A. F. Ngomsik and A. Bee, Water Res., 2010, 44, 1683–1688 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03692a |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–80
000–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Beijing Chemical Factory. Triton-X 100 (C34H62O11) was purchased from Aldrich. Ammonium ferric citrate [Fe(NH4)3(C6H5O7)2] was purchased from Shantou Xilong Chemical Corporation. Potassium dichromate (K2Cr2O7) was purchased from Beijing Chemical Factory. All the reagents were used without further purification.
000) was purchased from Beijing Chemical Factory. Triton-X 100 (C34H62O11) was purchased from Aldrich. Ammonium ferric citrate [Fe(NH4)3(C6H5O7)2] was purchased from Shantou Xilong Chemical Corporation. Potassium dichromate (K2Cr2O7) was purchased from Beijing Chemical Factory. All the reagents were used without further purification.