K-bis(2-ethylhexyl) phosphate (BEHPK): a novel additive for C–H arylation†
Masahiko Seki*
Process R&D Department, Healthcare Business Division II, API Corporation, 2-3-4, Nihonbashi, Chuo-ku, Tokyo 103-0027, Japan. E-mail: seki.masahiko@mm.api-corp.co.jp
First published on 18th June 2014
Abstract
A Ru-mediated highly efficient direct C–H arylation of α-heteroaromatic benzenes has been accomplished by addition of readily accessible K-bis(2-ethylhexyl) phosphate (BEHPK), which allowed facile and economical access to a wide range of functionalized biaryls including active pharmaceutical ingredients.
Inspired by conceptually high atom economy, Ru-catalyzed C–H arylation has aroused a keen interest in the synthesis of biaryls (Fig. 1).1 The approach does not need any activating group such as boronic acid to effect the C(sp2)–C(sp2) coupling. However, most of the protocols hitherto reported required high Ru catalyst loading (5 to 10 mol%) and often suffered from lack of reproducibility in large scale synthesis.2 During our process development of angiotensin II receptor blockers (ARBs) using C–H arylation,3 the same kind of issues have arisen in the reaction of 1-benzyl-2-phenyl-1H-tetrazole (1) with 4-bromobenzyl acetate (2) whose product 3 is a key common intermediate for ARBs (Table 1, Entry 1).3 It seriously retarded further development to multi-hundred kilogram scale commercial production using the new approach. To address the challenge, we searched for a better catalytic system with a new additive to ensure robustness of the C–H arylation on a large scale. Disclosed herein is a highly efficient and practical protocol for the C–H arylation employing a novel additive, K-bis(2-ethylhexyl) phosphate (BEHPK).
| Entry | Additive | 3/4b | Conv.b (%) | Yieldc (%) |
|---|---|---|---|---|
| a Reactions were conducted by employing 1 (2.0 g, 8.46 mmol), 2 (2.13 g, 9.31 mmol, 1.1 equiv.), [RuCl2(p-cymene)]2 (26 mg, 0.0423 mmol, 0.5 mol%), PPh3 (44 mg, 0.168 mmol, 2.0 equiv. to Ru), additive (0.168 mmol, 2.0 equiv. to Ru), K2CO3 (1.17 g, 8.46 mmol, 1.0 equiv.) in NMP (10 mL) at 138 °C for 6 h.b Determined by HPLC.c Assay yield.d Not determined.e PPh3 (22 mg, 0.084 mmol, 1.0 equiv. to Ru) was employed. | ||||
| 1 | None | —d | 0–86 | 0–80 |
| 2e | AcOK | 89/11 | 51 | 39 |
| 3 | AcOK | 89/11 | 80 | 71 |
| 4 | PivOK | 86/14 | 71 | 64 |
| 5 | K-adamantanecarboxylate | 88/12 | 78 | 77 |
| 6 | K-mesitylenecarboxylate | 88/12 | 67 | 62 |
| 7 | (EtO)2P(O)(OK) | 94/6 | 62 | 56 |
| 8 | (BuO)2P(O)(OK) | 97/3 | 34 | 25 |
| 9 | (BuO)P(O)(OK)2 | 96/4 | 40 | 29 |
| 10 |  |
97/3 | 25 | 16 |
| 11 | (PhO)2P(O)(OK) | 93/7 | 66 | 60 |
| 12 | (2-Et-HexO)2P(O)(OK) (BEHPK) | 95/5 | 87 | 82 |
Lack of reproducibility in the Ru-catalyzed C–H arylation has been reported by Merck in the synthesis of Anacetrapib.2 They analysed a trace amount of impurities in the reaction system and eventually found addition of a carboxylic acid salt (AcOK (ref. 4)) was effective to make the reaction reproducible. Hence, we first tested AcOK as an additive for the C–H arylation. The reaction was conducted by stirring a mixture of 1 and 2 in the presence of [RuCl2(p-cymene)]2 (0.5 mol%) and PPh3 (1.0 equiv. to Ru) and K2CO3 in NMP at 138 °C for 6 h. A poor yield was obtained and ratio of desired monoarylation product 3 was moderate due to considerable formation of diarylation product 4 (3/4 = 89/11, 39% yield, Table 1, Entry 2). To improve the yield the amount of PPh3 was doubled (2.0 equiv. to Ru). Although a better yield was obtained, selectivity of the monoarylation was not improved (3/4 = 89/11, 71% yield, Table 1, Entry 3). Use of other well documented carboxylates for the C–H arylation such PivOK,5 K-adamantanecarboxylate6 and K-mesitylenecarboxylate6 was similarly accompanied by appreciable magnitude of diarylation (3/4 = 86/14 to 88/12, Table 1, Entries 4–6). The diarylation product 4 might be removed by recrystallization. However, if the final product is an active pharmaceutical ingredient (API), to reduce the extent of diarylation is quite significant to confirm high quality of the product by conducting minimum number of purification.7 The diarylation product might be carried over to the final stage and contaminate API.
To work out the issue, we came up with an idea employing K-phosphate as the additive. In situ generated Ru-phosphate might facilitate removal of C–H hydrogen by Lewis basic P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxygen (Fig. 2).8 Two substituents (R) on the phosphate are able to be modified readily to enhance the basicity of the P
O oxygen (Fig. 2).8 Two substituents (R) on the phosphate are able to be modified readily to enhance the basicity of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxygen by inductive effect and/or to retard the diarylation by steric repulsion.
O oxygen by inductive effect and/or to retard the diarylation by steric repulsion.
The reaction with a K-phosphate additive was first tested by the use of K-diethyl phosphate. As expected, monoarylation selectivity was much improved but the yield became lower (3/4 = 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 56% yield, Table 1, Entry 7). More sterically demanding K-dibutyl phosphate provided a much poorer yield while di-K-monobutyl phosphate gave a similar outcome (25% and 29% yield, respectively, Table 1, Entries 8 and 9). Cyclic K-binaphtyl phosphate9 did not improve the yield (16%, Table 1, Entry 10). By using diphenyl phosphate, the yield became higher though still being unsatisfactory (60% yield, Table 1, Entry 11). It should be noted that monoarylation selectivity is high without exception when the phosphates were employed. To improve the yield, a more electronegative aliphatic branched K-bis(2-ethylhexyl) phosphate (BEHPK) was then tested. Gratifyingly, using BEHPK as an additive, a much higher yield was obtained while monoarylation selectivity was retained (3/4 = 95
6, 56% yield, Table 1, Entry 7). More sterically demanding K-dibutyl phosphate provided a much poorer yield while di-K-monobutyl phosphate gave a similar outcome (25% and 29% yield, respectively, Table 1, Entries 8 and 9). Cyclic K-binaphtyl phosphate9 did not improve the yield (16%, Table 1, Entry 10). By using diphenyl phosphate, the yield became higher though still being unsatisfactory (60% yield, Table 1, Entry 11). It should be noted that monoarylation selectivity is high without exception when the phosphates were employed. To improve the yield, a more electronegative aliphatic branched K-bis(2-ethylhexyl) phosphate (BEHPK) was then tested. Gratifyingly, using BEHPK as an additive, a much higher yield was obtained while monoarylation selectivity was retained (3/4 = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 82% yield, Table 1, Entry 12). BEHPK is an inexpensive and widely available chemical and hence readily applicable to commercial large scale production.
5, 82% yield, Table 1, Entry 12). BEHPK is an inexpensive and widely available chemical and hence readily applicable to commercial large scale production.
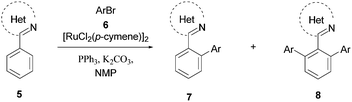
Scope of the reaction was tested employing various arenes 5 as the substrate (Table 2). The reaction was conducted in the presence of 2.2 equiv. of ArBr 6 and 0.5 mol% of [RuCl2(p-cymene)]2. The C–H arylation took place smoothly for 2-phenylpyridine to give arylated product 7 and 8 in good yields though the monoarylation selectivity was poor (Table 2, Entries 1–4). The reaction of 1-phenylpyrazole and 2-phenyloxazoline smoothly provided corresponding arylated products as well (Table 2, Entries 5–10). When 1-benzyl-5-phenyl-1H-tetarazole was reacted with 4-brormotoluene or 4-bromobenzyl benzoate, the reaction proceeded well to give the monoarylation product selectively (Table 2, Entries 11 and 12). Although the monoarylation selectivity was not achieved in the reactions except 1-benzyl-5-phenyl-1H-tetarazole as the substrate, fairly good yields were obtained in the presence of quite low Ru catalyst loading (0.5 mol%).10
| Entry | Arene 5 | ArBr 6 | 7/8 | Conv.b (%) | Yieldc (%) |
|---|---|---|---|---|---|
| a Reactions were conducted by employing 5 (8.46 mmol), 6 (18.6 mmol, 2.2 equiv.), [RuCl2(p-cymene)]2 (26 mg, 0.0423 mmol, 0.5 mol%), PPh3 (44 mg, 0.168 mmol, 2.0 equiv. to Ru), BEHPK (0.168 mmol, 2.0 equiv. to Ru), K2CO3 (1.17 g, 8.46 mmol, 1.0 equiv.) in NMP (10 mL) at 138 °C for 6 h.b Determined by HPLC.c Assay yield.d The amount of bromide 6 is 1.1 equiv. | |||||
| 1 |  |
4-BrPhMe | 5/95 | 100 | 2 (7) |
| 97 (8) | |||||
| 2 | 4-BrPhCO2Me | 4/96 | 100 | 1 (7) | |
| 99 (8) | |||||
| 3 | 4-BrPhCH2OAc | 0/100 | 100 | 93 (8) | |
| 4 | 4-BrPhCH2OBz | 31/69 | 100 | 28 (7) | |
| 61 (8) | |||||
| 5 |  |
4-BrPhMe | 21/79 | 48 | 10 (7) |
| 38 (8) | |||||
| 6 | 4-BrPhCO2Me | 8/92 | 100 | 8 (7) | |
| 85 (8) | |||||
| 7 | 4-BrPhCH2OAc | 1/99 | 100 | 1 (7) | |
| 94 (8) | |||||
| 8 | 4-BrPhCH2OBz | 12/88 | 91 | 12 (7) | |
| 88 (8) | |||||
| 9 |  |
4-BrPhMe | 21/30 | 51 | 17 (7) |
| 26 (8) | |||||
| 10 | 4-BrPhCH2OAc | 31/69 | 45 | 14 (7) | |
| 31 (8) | |||||
| 11d |  |
4-BrPhMe | 95/5 | 81 | 77 (7) |
| 3 (8) | |||||
| 12d | 4-BrPhCH2OBz | 92/8 | 77 | 68 (7) | |
| 4 (8) | |||||
In conclusion, BEHPK was found to be highly effective for C–H arylation. It was applied to a practical synthesis of a key common intermediate for ARBs. In the C–H arylation of 1-benzyl-5-phenyl-1H-tetarazole using BEHPK as the additive, higher monoarylation selectivity was achieved to provide the product of much higher purity. Using BEHPK, consistency of the reaction in scale was observed as well.11 Ready availability of BEHPK and ease of operation of the new process would permit a facile access to biaryls of pharmaceutical and commercial importance.
Notes and references
- For reviews on Ru-catalyzed C–H arylation, see: (a) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (b) L. Ackermann, Chem. Commun., 2010, 46, 4866 RSC; (c) L. Ackermann and R. Vicente, Top. Curr. Chem., 2010, 292, 211 CrossRef CAS; (d) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (e) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed.
- S. G. Ouellet, A. Roy, C. Molinaro, R. Angelaud, J. F. Marcoux, P. D. O'Shea and J. W. Davies, J. Org. Chem., 2011, 76, 1436 CrossRef CAS PubMed.
- (a) M. Seki, ACS Catal., 2011, 1, 607 CrossRef CAS; (b) M. Seki and M. Nagahama, J. Org. Chem., 2011, 76, 10198 CrossRef CAS PubMed; (c) M. Seki, Synthesis, 2012, 44, 3231 CrossRef CAS PubMed; (d) M. Seki, J. Syn. Org. Chem. Jpn., 2012, 70, 1295 CrossRef CAS; (e) P. Kocienski, Synfacts, 2013, 9, 0006 Search PubMed; (f) E. Dier, N. Y. P. Kumar, T. Mejuch, I. Marek and L. Ackermann, Tetrahedron, 2013, 69, 4445 CrossRef PubMed.
- (a) F. Pozgan and P. H. Dixneuf, Adv. Synth. Catal., 2009, 351, 1737 CrossRef CAS; (b) B. Li, C. B. Bheeter, C. Darcel and P. H. Dixneuf, ACS Catal., 2011, 1, 1221 CrossRef CAS.
- (a) P. B. Arockiam, V. Poirier, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Green Chem., 2009, 11, 1871 RSC; (b) P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Angew. Chem., Int. Ed., 2010, 49, 6629 CrossRef CAS PubMed.
- L. Ackermann and A. V. Lygin, Org. Lett., 2011, 13, 3332 CrossRef CAS PubMed.
- N. G. Anderson, in Practical Process Research & Development, ed. Academic Press, Oxford, 2000 Search PubMed.
- (a) T. Honjo, R. J. Phipps, V. Rauniyar and F. D. Toste, Angew. Chem., Int. Ed., 2012, 51, 9684 CrossRef CAS PubMed; (b) L. Liu, T. Fu, T. Wang, X. Gao, Z. Zeng, J. Zhu and Y. Zhao, J. Org. Chem., 2014, 79, 80 CrossRef CAS PubMed; (c) K. M. Blażewska, J. Org. Chem., 2014, 79, 408 CrossRef PubMed.
- The Ru(II)-phosphate derived from cyclic K-binaphtyl phosphate was successfully applied to alkenylation of aryloxazoline: B. Li, K. Devaraj, C. Darcel and P. H. Dixneuf, Green Chem., 2012, 14, 2706 RSC.
- High monoarylation selectivity observed for 1-benzyl-5-phenyl-1H-tetarazole was owing to the presence of sterically demanding 1-benzyl group.3 High monoarylation selectivity for other substrates was reported by the use of RuCl2(PPh3)(p-cymene): P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Green Chem., 2013, 15, 67 RSC . However, reactivity of the catalyst was poor toward the substrate 1 and price of the catalyst is much higher than that of [RuCl2(p-cymene)]2. Use of water as the solvent was not explored in this study.
- The reaction of 1 with 4-bromobenzylbenzoate (Table 2, Entry 12) was successfully conducted with high reproducibility employing 500 g of 1.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c4ra03679d |
| This journal is © The Royal Society of Chemistry 2014 |