Adsorption of metal ions by clays and inorganic solids
Susmita Sen Gupta
a and
Krishna G. Bhattacharyya
*b
aDepartment of Chemistry, B N College, Dhubri 783324, Assam, India
bDepartment of Chemistry, Gauhati University, Guwahati 781014, Assam, India. E-mail: kgbhattacharyya@gmail.com; Tel: +91 9864031987
First published on 12th June 2014
Abstract
This review deals with adsorption of metal ions, particularly those considered as hazardous, on clays and some inorganic solids and covers the publication years 2000–2013 describing and quantifying the use of isotherms to obtain the adsorption capacities of the solids. The inorganic solids in the review include clays and clay minerals, their modified forms (obtained by treatment with acids and alkalis, organic functionalization, etc.), zeolites, silica gel, soil, river sediment, activated alumina, inorganic polymers, red mud, inorganic oxides, fly ash, etc. The use of two parameter and three parameter linear isotherms are only discussed with a view to obtain quantitative description of adsorptive accumulation and separation of metal ions from aqueous solution on the solids. The extensively used isotherms are those of Freundlich, Langmuir, Dubinin–Radushkevich and Redlich–Peterson. How these isotherms are being used to obtain the adsorption capacities along with their interpretations form the bulk of the review. The review is divided into sections, each describing the use of a particular isotherm. The metal cations receiving immense importance in adsorption studies are As(III)/As(V), Cd(II), Cr(III)/Cr(VI), Cu(II), Co(II), Pb(II), Ni(II), Hg(II), and Zn(II), and the review covers both the cationic and the anionic metal ions. A few other cations such as Mn(II), Fe(III), Se(V), which have not received much attention, are also covered.
1. Introduction
Inorganic solids like oxides, clay minerals, zeolites, etc., play a leading role in maintaining environmental purity by adsorbing and separating undesirable components from the liquid phase. Equilibrium relationships between the quantity adsorbed in the solid phase and that remaining unadsorbed in the solution at a fixed temperature help in evaluating the adsorption capacities of the solids.1 The equilibrium relationship illustrates how a constituent in the liquid phase interacts with the solid adsorbent and how the adsorption mechanism can be optimised for practical applications.2,3 The isotherms are often used to predict modeling procedures for analysis and design of a particular system and for theoretical evaluation and interpretation of thermodynamic parameters.4The equilibrium isotherm yields a range of quantitative information about the performance of a solid5 such as the absorbability, amount adsorbed per unit mass, maximum amount that can be removed from solution, the sensitivity of the adsorbent to changes in adsorbate concentration, etc. The isotherm suffers from the limitation that it is strictly applicable to equilibrium conditions without any time restriction, and hence if true equilibrium is difficult to ascertain, the applicability of the isotherm will remain uncertain. Further, long-term chemical and biological effects are not accounted for by the isotherm.
A solid is a good adsorbent if it has high internal volume accessible to the adsorbate molecules or ions. Surface area, particularly the internal pore surface and pore size distribution determine the extent of adsorption. How quickly the adsorbing molecules reach the adsorption sites is another important criterion for consideration.6 The physical size and form of the solid particles, the chemistry of the adsorbent, namely, degree of ionization at the surface, the types of functional groups present and the degree to which these properties change in contact with an aqueous solution are important considerations in determining the adsorption capacity of a solid.
During the last few years, a good number of reviews have appeared on water treatment through adsorption. A few of these reviews include (i) arsenic remediation by different types of adsorbents,7 (ii) advanced adsorption methods that specialize in the composition, structure, function and characteristics of the solids for water treatment,8 (iii) use of low cost inorganic and biomass-based adsorbents for wastewater treatment,9 (iv) utilization of agro-industrial and municipal wastes for detoxification of water,10 (v) natural and modified zeolites as adsorbents for water and wastewater treatment,11 (vi) natural and modified kaolinite and montmorillonite as adsorbents for metal ions removal,12–14 (vii) development and potential applications of adsorption isotherms,3 etc.
The present work reviews adsorption of metal cations and anions on inorganic solids since 2000 and the use of isotherms to characterize the solids in terms of the adsorption capacities. The inorganic adsorbents included in this review are clays and clay minerals, zeolites, silica gel, soil, river sediment, activated alumina, inorganic polymer, red mud, inorganic oxides (viz., hydrous zirconium oxide, titanium oxide, stannic oxide, ferric oxide), fly ash, etc. These substances are used as they are found in nature and also modified chemically through various treatments. Table 1 summarises a list of such inorganic adsorbents with their compositions.
| Absorbent | Composition | Ion exchange capacity (mmol per 100 g) | Ref. |
|---|---|---|---|
| Kaolin | SiO2 (46.70%), Al2O3 (37.33%), TiO2 (<0.01%), Fe2O3 (0.75%), Na2O (<0.10%), K2O (0.93%), CaO (<0.10%), MgO (<0.10%), MnO (0.04%) | 11.3 | 27 |
| Ballclay | SiO2 (62.96%), Al2O3 (22.61%), TiO2 (0.51%), Fe2O3 (1.49%), Na2O (<0.10%), K2O (2.01%), CaO (0.51%), MgO (0.51%), MnO (0.02%) | 14.9 | |
| Turkish kaolinite | SiO2 (59.46%), Al2O3 (14.92%), K2O (2.45%), MgO (1.98%), Fe2O3 (5.18%), CaO (4.75%), SO3 (0.10%), Na2O (0.98%), TiO2 (0.73%), Kuvars (quartz) (16.64%), calcite (8.14%), anorthite (11.53%), illite (42.58%), chlorite (21.11%) | — | 31 |
| Montmorillonite K-10 | SiO2 (65.34%), Al2O3 (12.89%), Fe2O3 (2.38%), MgO (0.95%), TiO2 (0.52%), CaO (0.24%), Na2O (0.53%), K2O (1.54%), P2O5 (7.85%) | 45.1 | 33 |
| Natural Brazilian bentonite | SiO2 (57.85%), Al2O3 (16.74%), Fe2O3 (8.78%), MgO (2.73%), TiO2 (1.24%), CaO (1.08%), Na2O (0.73%), K2O (0.43%), P2O5 (0.27%) | 74.8 | |
| Turkish bentonite | SiO2 (71.90%), Al2O3 (13.85%), Fe2O3 (0.68%), TiO2 (0.09%), CaO (2.42%), MgO (1.27%), Na2O (0.39%), K2O (1.62%) | 45.5 (pH 5.0) | 34 |
| 51.9 (pH 6.4) | |||
| 62.4 (pH 8.0) | |||
| Sepiolite | SiO2 (58.7%), MgO (25.0%), Al2O3 (0.5%), CaO (0.5%), FeO + Fe2O3 (0.05%), TiO2 (0.05%), Na2O (0.05%) | — | 151 |
| Vermiculite | SiO2 (39.0%), MgO (20.0%), Al2O3 (12.0%), Fe2O3 (8.0%), K2O (4.0%), CaO (3.0%) | 100.0 | 90 |
| Laterite | Fe-oxide (47.26%), SiO2 (28.67%), aloxide (21.13%), MnO2 (0.78%), Na2O (0.69%) | — | 208 |
| Muscovite | SiO2 (61.32%), Al2O3 (23.90%), K2O (7.12%), CaO (2.48%), MgO (2.07%), Fe2O3 (1.31%), TiO2 (0.26%), Na2O (0.15%) | 0.3 | 43 |
| Perlite | SiO2, Al, K, Na | — | 161 |
| Attapulgite | C (2.63%), H (1.12%), N (9.65%) | — | 132 |
| Tourmaline | SiO2 (41.56%), Al2O3 (26.3%), Fe2O3 (12.19%), TiO2 (0.26%), B2O3 (9.33%), FeO (5.4%), CaO (0.52%), MgO (0.55%), K2O (0.14%), Na2O (1.2%), P2O5 (0.22%), Fe2O3 (1.59%), LiO2 (1.87%), MnO (0.04%), F (1.06%) | 384.0 | 122 |
| Dolomite | MgCO3 (44.0%), CaCO3 (53.0%), (SiO2 and Fe as impurities) | — | 205 |
| Beidellite | SiO2 (58.08%), Al2O3 (29.92%), MgO (5.48%), Fe2O3 (2.96%), Na2O (1.85%), CaO (0.63%), K2O (0.2%) | — | 181 |
| Natural clinoptilolite | SiO2 (69.12%), Al2O3 (12.05%), CaO (2.23%), K2O (3.61%), Na2O (0.32%), Fe2O (0.98%), MgO (1.30%) | 190.0–220.0 | 85 |
| Zeolite | SiO2 (46.5%), MgO (2.3%), Al2O3 (15%), Fe2O3 (3%), K2O (6%), CaO (10%), Na2O2 (0.6%), TiO2 (0.3%) | 170.0 | 90 |
| Natural zeolitic volcanic tuff | SiO2 (69.57%), Al2O3 (10.59%), CaO (2.23%), K2O (5.37%), Na2O (1.50%), Fe2O3 (1.33%), MgO (0.77%), TiO2 (0.22%) | — | 82 |
| Low-grade phosphate | P2O5 (26.35%), CaO (48.13%), SiO2 (10.04%), CO2 (8.64%), Cl− (360 ppm), organic carbon (0.17%), moisture (1.32%) | — | 209 |
| Pumice | SiO2 (72.0%), MgO (0.1%), Al2O3 (11.9%), Fe2O3 (2.1%), K2O (5.1%), CaO (0.6%), TiO2 (0.1%) | 30.0 | 90 |
| Polymetallic sea nodule | MnO2 (31.8%), Fe2O3 (21.2%), SiO2 (14.2%) with traces of Cu, Ni, Co, Ca, K, Na and Mg | — | 222 |
| Vanadium mine tailing | C (28.47%), O (36.2%), Mg (0.88 %), Al (5.99%), Si (21.1%), K (2.48%), V (1.45%), Fe (1.09%) | — | 223 |
| Activated alumina | SiO2 (0.03 %), Fe2O3 (0.03 %), Al2O3 (93.1 %), Na2O (0.1 %) | — | 111 |
| Loess soil | SiO2 (63.68%), Al2O3 (12.77%), CaO (9.56%), MgO (3.14%), K2O (3.01%), Fe2O3 (2.74%), Na2O (2.35%), FeO (0.89%), TiO2 (0.78%), MnO (0.09%) | 11.2 | 123 |
| Fly ash (sugar industry) | SiO2 (60.5%), Al2O3 (15.4%), CaO (2.90%), Fe2O3 (4.90%), MgO (0.81%) | — | 231 |
| Fly ash (thermal power plant) | SiO2 (52.0%), Al2O3 (27.0%), Fe2O3 (6.0%), MgO (0.70%), CaO (0.11%), TiO2 (1.01%) | — | 235 |
| Pit coal fly ash | SiO2 (47.39%), Al2O3 (23.49%), Fe2O3 (8.55%), CaO (4.67%), Na2O (1.36%) | — | 233 |
| Clarified sludge | SiO2 (12.6%), Fe2O3 (48.0%), CaO (23.4%), MgO (2.5%), MnO (0.2%), Na2O (0.7%), K2O (0.5%) | — | 239 |
| Fe2O3 (48.0%), CaO (23.4%), MgO (2.5%), MnO (0.2%), SiO2 (12.6%), Na2O (0.7%), K2O (0.5%) | — | 240 | |
| Red mud | SiO2 (9.64%), Al2O3 (17.28%), Fe2O3 (38.80%), TiO2 (18.80%), Na2O (6.86%) | — | 237 |
| Electric furnace slag | SiO2 (27.54%), Fe2O3 (0.49%), FeO (7.11%), CaO (26.74%), MgO (22.84%), Mn2O3 (9.71%), Al2O3 (4.52%), P2O5 (0.019%), S (0.015%), K2O (0.60%), Na2O (0.23%) | — | 137 |
2. Quantitative treatment of the adsorption equilibrium
Adsorption is described as one of the fascinating phenomena related with the behaviour of fluids in a force field exerted by the solid surface.15 While chemical equilibrium in a homogeneous system is driven by a reduction of the free energies of the bulk system, the same for a heterogeneous surface reaction comes from a reduction in the surface energy.In formulating an adsorption isotherm, three basic approaches are followed, namely, (i) consideration of adsorption as a state of dynamic equilibrium involving both adsorption and desorption,16 (ii) thermodynamic treatment of adsorption that provides a framework to obtain a number of isotherm models17,18 and (iii) potential energy profiles of the adsorbate as it comes close to the adsorbent.19 In many cases, more than one approach is utilised in obtaining an isotherm that makes it possible to interpret the adsorption phenomenon.20
This review considers the use of linear isotherms to obtain adsorption capacities of clays and other inorganic solids with respect to adsorptive accumulation and separation of metal ions from aqueous solution. Freundlich and Langmuir isotherms continue to be most extensively and inbariably used by all adsorption scientists, while Dubinin–Radushkevich, Redlich–Peterson, Temkin and Toth isotherms have also been explored in a number of works. The estimation of the adsorption capacities of clays and other inorganic solids based on these isotherms form the bulk of this review. There are, however, wide disparities in the nomenclature and the units used for expressing the adsorption capacities and other coefficients (including no use of units in some cases) and the review is therefore confined to those cases where these entities are well defined and are unambiguous. Molecular simulation studies, particularly with respect to adsorption isotherms and adsorbate–adsorbent interactions, have also been referred to in this review.
2.1. Freundlich isotherm and its applications
The Freundlich isotherm,21 one of the most widely used adsorption equilibrium model, started on an empirical ground, but was shown latter to be thermodynamically rigorous for adsorption on heterogeneous surfaces.22 Any non-uniform distribution of adsorption heat and affinities over a heterogeneous surface leading to multilayer adsorption can be described with this isotherm.23 Freundlich model proposed that the stronger binding sites on a solid surface were occupied first until adsorption energy decreased to a value when no further adsorbate–adsorbent interactions were possible.3,24,25The Freundlich isotherm and its linearized form,
| qe = KFCen | (1) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + n KF + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (2) |
| Metal cation/adsorbent | Experimental variables | Freundlich capacity (KF) | Ref. | ||
|---|---|---|---|---|---|
| As(III) | |||||
| MnFe2O4 | 293 K | pH 7.0 | 29.6 | mg1−1/n L1/n g−1 | 104 |
| CoFe2O4 | 36.9 | ||||
| Fe3O4 | 15.2 | ||||
| Natural muscovite | 293 K | As(III) 0–100 mg L−1, pH 6.0 | 0.029 | mg1−1/n L1/n g−1 | 43 |
| As(V) | |||||
| Natural muscovite | 293 K | As(V) 0–100 mg L−1, pH 6.0 | 0.200 | mg1−1/n L1/n g−1 | 43 |
| Laterite | 305 K | As(V) 0.5–2.0 mg L−1, pH 5.5 | 0.498 | mg L−1 | 208 |
| As(V) 2.0–20.0 mg L−1, pH 5.5 | 0.147 | ||||
| As(V) 0.2–20.0 mg L−1, pH 7.0 | 0.234 | ||||
| As(V) 3.0–20.0 mg L−1, pH 7.0 | 0.124 | ||||
| As(V) 0.2–20.0 mg L−1, pH 5.5 | 0.306 | ||||
| 293 K | As(V) 0.5–2.0 mg L−1, pH 7.0 | 0.124 | |||
| As(V) 2.0–20.0 mg L−1, pH 7.0 | 0.105 | ||||
| 315 K | As(V) 0.5–2.0 mg L−1, pH 5.5 | 0.665 | |||
| As(V) 2.0–20.0 mg L−1, pH 5.5 | 0.245 | ||||
| As(V) 0.5–2.5 mg L−1, pH 7.0 | 0.174 | ||||
| As(V) 2.5–20.0 mg L−1, pH 7.0 | 0.144 | ||||
| As(V) 3.5–20.0 mg L−1, pH 5.5 | 0.381 | ||||
| MnFe2O4 | 293 K | pH 3.0 | 59.7 | mg1−1/n L1/n g−1 | 104 |
| CoFe2O4 | 49.4 | ||||
| Fe3O4 | 19.2 | ||||
| Fe modified beydellite | 298 K | As(III) 250–5000 μg L−1 | 26.46 | μg g−1 | 181 |
| 308 K | 36.79 | ||||
| 318 K | 45.63 | ||||
| Fe–Ce modified zeolite | 298 K | 10.40 | |||
| 308 K | 11.51 | ||||
| 318 K | 15.05 | ||||
| Fe modified sepiolite | 298 K | 7.98 | |||
| 308 K | 10.34 | ||||
| 318 K | 11.21 | ||||
| Iron oxide-coated perlite | 303 K | pH 6.5–7.0 | 0.76 | L mg−1 | 161 |
| Al-loaded-zeolite | 297 K | As(V) 1.3 mμ | 0.122 | mmol g−1 | 86 |
| 0.088 | |||||
| Chitosan–clay–magnetite composite | 296 ± 2 K | pH 5.0 | 0.282 | L g−1 | 178 |
| Activated red mud | 288 K | As(V) 5.74–182.57 μM, pH 7.0 ± 0.1 | 5.93 | L μmol−1 | 138 |
| 293 K | As(V) 7.70–136.50 μM, pH 7.0 ± 0.1 | 6.71 | |||
| 296 ± 1 K | As(V) 2.0–151.87 μM, pH 7.0 ± 0.1 | 17.98 | |||
| 323 K | As(V) 6.91–152.18 μM, pH 7.0 ± 0.1 | 16.85 | |||
| 296 ± 1 K | As(V) 7.03–220.85 μM, pH 4.5 ± 0.1 | 63.29 | |||
| Cd(II) | |||||
| Kaolin | 303 K | 6.22 × 10−4 | mmol g−1 | 27 | |
| Ball clay | 3.11 × 10−3 | ||||
| Bentonite | 303 K | Cd(II) 50–300 mg L−1 | 46.56 | mg g−1 | 36 |
| Montmorillonite K 10 | 298 K | 0.42 | mg g−1 | 33 | |
| Brazilian bentonite | 1.74 | ||||
| Acid-activated kaolinite | 303 K | pH 5.5 | 0.80 | mg g−1 | 46 |
| Acid-activated montmorillonite | 12.90 | ||||
| Montmorillonite | 293 K | 3775.5 | μM1−n kg−1 Ln | 26 | |
| Hydroxy-Al interlayered montmorillonite | 507.0 | ||||
| Fe-pillared bentonite | Cd(II) 0.178–5.34 mmol L−1 | 0.451 | L g−1 | 63 | |
| Fe/Cr-pillared bentonite | 0.347 | ||||
| Cr-pillared bentonite | 0.264 | mg1−1/n L1/n g−1 | 47 | ||
| Kaolinite | 303 K | pH 5.5 | 0.38 | ||
| ZrO-kaolinite | 0.33 | ||||
| TBA-kaolinite | 0.32 | ||||
| Montmorillonite | 6.76 | ||||
| ZrO-montmorillonite | 1.20 | ||||
| TBA-montmorillonite | 2.21 | ||||
| Bentonite | 303 K | pH 6.02 | 0.82 | mg g−1 | 58 |
| 323 K | 2.40 | ||||
| Bentonite + goethite | 303 K | 0.70 | |||
| 323 K | 1.08 | ||||
| Bentonite + humic acid | 303 K | 1.89 | |||
| 323 K | 2.82 | ||||
| Bentonite + geothite + humic acid | 303 K | 1.61 | |||
| 323 K | 2.65 | ||||
| Goethite | 300 ± 1 K | Cd(II) 5–25 mg L−1 | 2.140 | L g−1 | 113 |
| Boehmite (γ-AlOOH) | 298 K | Cd(II) 50 mg L−1, pH 6.0 | 0.111 | mg1−1/n L1/n g−1 | 114 |
| Goethite (γ-FeOOH) | 0.074 | ||||
| Vermiculite | 298 K | Cd(II) 0–500 μM | 0.65 | L g−1 | 90 |
| Zeolite | 0.55 | ||||
| Pumice | 0.69 | ||||
| Natural muscovite | 293 K | Cd(II) 0–100 mg L−1, pH 6.0 | 0.007 | mg1−1/n L1/n g−1 | 43 |
| Zeolite-based geopolymer | 6.603 | L g−1 | 89 | ||
| NiO | 303 K | Cd(II) 100–600 mg L−1 | 6.616 | mg g−1 | 201 |
| Hydrous MnO2 | 298 K | pH 3.5–4.0 | 1.367 | mmol1−n Ln g−1 | 96 |
| Iron oxide-immobilized sand | 298 ± 1 K | 0.179 | mg g−1 | 128 | |
| Thiol-functionalized silica | 298 K | Cd(II) 20–250 mg L−1 | 25.65 ± 1.43 | L g−1 | 117 |
| Loess soils | 278 K | Cd(II) 25–150 mg L−1 | 8.80 | mg g−1 | 123 |
| 288 K | 4.02 | ||||
| 298 K | 4.05 | ||||
| 308 K | 4.26 | ||||
| 318 K | 4.87 | ||||
| Titanate nanotube | 298 ± 1 K | Cd(II) 0.1–3.0 mmol L−1 | 0.92 | mmol g−1 | 80 |
| Silico-antimonate ion exchanger | 298 ± 1 K | 0.79 | mmol g−1 | 133 | |
| Activated alumina | 303 K | pH 5.0 | 4.34 | mg1−1/n L1/n g−1 | 111 |
| Clarified sludge | 303 ± 0.5 K | pH 5.0 | 4.37 | mg1−1/n L1/n g−1 | 240 |
| Dithiocarbamate chelating resin | 298 K | Cd(II) 1–25 mmol L−1 | 0.831 | mmol L−1 | 136 |
| Co(II) | |||||
| Bentonite | 293 K | pH 3.0 | 0.460 | mg g−1 | 34 |
| pH 5.0 | 1.093 | ||||
| pH 7.0 | 2.290 | ||||
| pH 9.0 | 3.834 | ||||
| Kaolinite | 303 K | pH 5.8 | 1.1 | mg1−1/n L1/n g−1 | 50 |
| Acid-activated kaolinite | 1.5 | ||||
| Montmorillonite | 4.6 | ||||
| Acid-activated montmorillonite | 6.0 | ||||
| ZrO-kaolinite | 303 K | Co(II) 10–250 mg L−1, pH 5.7 | 0.8 | mg1−1/n L1/n g−1 | 49 |
| ZrO-montmorillonite | 1.2 | ||||
| HCl/NaOH-treated bentonite | 295 ± 1 K | Co(II) 20–200 mg L−1 | 1.02 | mg g−1 | 45 |
| Ion exchange resins | IRN77 | 298 ± 1 K | 75.63 | mg g−1 | 135 |
| SKN1 | 60.03 | ||||
| Cr(III) | |||||
| Kaolin | 303 K | 1.13 × 10−3 | mmol g−1 | 27 | |
| Acid-activated kaolinite | 1.5 | ||||
| ZrO-kaolinite | 1.0 | ||||
| TBA-kaolinite | 0.9 | ||||
| Tannin-immobilized activated clay | 300 K | 2.406 | L g−1 | 76 | |
| 310 K | 2.670 | ||||
| 320 K | 2.687 | ||||
| 330 K | 3.113 | ||||
| [3-(2-Aminoethylamino)propyl]trimethoxy-silane grafted sepiolite | 2.042 | mg1−1/n g−1 L1/n | 78 | ||
| [3-(2-Aminoethylamino)propyl]trimethoxy-silane grafted acid-activated sepiolite | 2.845 | ||||
| Mercapto functionalized sepiolite | 289.15 K | Pb(II) 0–80 mg L−1 | 21.89 ± 1.97 | mg g−1 | 79 |
| 299.15 K | 22.90 ± 2.00 | ||||
| 318.15 K | 33.73 ± 1.28 | ||||
| Zero-valent iron | 298 K | Cr(VI) 60–100 mg L−1 | 56.88 | mg g−1 | 62 |
| Fe–Ni bimetallic nanoparticle | 64.71 | ||||
| Fe–Ni bimetallic-montmorillonite nanocomposite | 69.13 | ||||
| Hydrous TiO2 | 288 K | pH 1.5 | 4.067 | mg g−1 | 255 |
| 303 K | 8.241 | ||||
| 318 K | 7.804 | ||||
| Hydrous ZrO2 | 298 K | pH 2.0 | 7.05 | mg g−1 | 91 |
| 308 K | 6.97 | ||||
| 318 K | 6.51 | ||||
| 328 K | 5.12 | ||||
| 338 K | 4.94 | ||||
| Loamy sand soil | 1078 | mg1−1/n L1/n kg−1 | 124 | ||
| Cu(II) | |||||
| Local bentonite | 293.15 K | 5.77 × 10−4 | mol1−n Ln g−1 | 37 | |
| 313.15 K | 6.33 × 10−4 | ||||
| 333.15 K | 5.91 × 10−4 | ||||
| Kaolin | 303 K | 4.20 × 10−4 | mmol g−1 | 27 | |
| Ball clay | 7.16 × 10−3 | ||||
| Bentonite | 303 K | Cu(II) 50–300 mg L−1 | 19.54 | mg g−1 | 36 |
| Bentonite | 293 K | pH 3.0 | 1.020 | mg g−1 | 34 |
| pH 5.0 | 1.622 | ||||
| pH 7.0 | 5.755 | ||||
| pH 9.0 | 8.482 | ||||
| Bentonite | 303 K | pH 6.02 | 0.84 | mg g−1 | 58 |
| 323 K | 2.87 | ||||
| Bentonite + goethite | 303 K | 1.00 | |||
| 323 K | 2.76 | ||||
| Bentonite + humic acid | 303 K | 1.92 | |||
| 323 K | 3.13 | ||||
| Bentonite + geothite + humic acid | 303 K | 1.38 | |||
| 323 K | 2.11 | ||||
| MgO coated bentonite | 295.15 K | pH 6.0 | 8.93 ± 0.90 | mg1−1/n L−1 g−1 | 59 |
| Kaolinite | 303 K | Cu(II) 10–250 mg L−1, pH 5.7 | 1.1 | mg1−1/n L1/n g−1 | 57 |
| Acid-activated kaolinite | 1.3 | ||||
| Montmorillonite | 9.2 | ||||
| Acid-activated montmorillonite | 12.4 | ||||
| 8-Hydroxy quinoline immobilized bentonite | 293 K | 34.78 | L g−1 | 70 | |
| 303 K | 35.83 | ||||
| 313 K | 37.86 | ||||
| 323 K | 41.16 | ||||
| 2,2′-Dipyridyl immobilized bentonite | 293 K | Cu(II) 92.5–200 mg L−1, pH 5.7 | 11.20 | L g−1 | 72 |
| 303 K | 20.72 | ||||
| 313 K | 23.85 | ||||
| 323 K | 30.02 | ||||
| Goethite | 300 ± 1 K | Cu(II) 5–25 mg L−1 | 2.227 | L g−1 | 113 |
| Natural muscovite | 293 K | Cu(II) 0–100 mg L−1, pH 6.0 | 0.034 | mg1−1/n L1/n g−1 | 43 |
| Chitosan–clay–magnetite composite | 296 ± 2 K | pH 5.0 | 5.828 | L g−1 | 178 |
| Iron oxide-immobilized sand | 298 ± 1 K | 0.487 | mg g−1 | 128 | |
Amino functionalized silica gel (3-aminopropyltri-methoxysilane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica = 0.1) silica = 0.1) |
1.416 | mg1−1/n L1/n g−1 | 115 | ||
Amino functionalized silica gel (3-aminopropyltri-methoxysilane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica = 0.2) silica = 0.2) |
5.77 | ||||
Amino functionalized silica gel (3-aminopropyltri-methoxysilane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica = 0.3) silica = 0.3) |
17.132 | ||||
| 3-Aminopropyltriethoxysilane and glutaraldehyde coated Fe3O4 | 293 ± 1 K | pH 4.0 | 14.544 | mmol1−1/n L1/n g−1 | 107 |
| Titanate nanotube | 298 ± 1 K | Cu(II) 0.1–3.0 mmol L−1 | 0.88 | mmol g−1 | 99 |
| Silico-antimonate ion exchanger | 298 ± 1 K | 0.44 | mmol g−1 | 133 | |
| Electric furnace slag | 293 K | 0.44 | mg1−1/n L1/n g−1 | 137 | |
| 303 K | 0.65 | ||||
| 313 K | 1.09 | ||||
| Fe(III) | |||||
| Kaolinite | 303 K | Fe(III) 10–250 mg L−1, pH 3.0 | 1.3 | mg1−1/n L1/n g−1 | 52 |
| Acid-activated kaolinite | 1.7 | ||||
| Montmorillonite | 5.2 | ||||
| Acid-activated montmorillonite | 6.4 | ||||
| ZrO-kaolinite | 303 K | Fe(III) 10–250 mg L−1, pH 3.0 | 1.0 | mg1−1/n L1/n g−1 | 49 |
| ZrO-montmorillonite | 1.4 | ||||
| Chitosan–attapulgite composites | 298 K | 7.402 | mg g−1 | 132 | |
| 308 K | 12.23 | ||||
| 318 K | 13.77 | ||||
| Hg(II) | |||||
| Hybrid mesoporous alumina-silicate sieve | 303 ± 2 K | Hg(II) 2–16 mg L−1 | 18.95 | mg g−1 | 80 |
| Dithiocarbamate chelating resin | 298 K | Hg(II) 1–25 mmol L−1 | 1.158 | mmol L−1 | 136 |
| Mn(II) | |||||
| Montmorillonite K 10 | 298 K | 0.31 | mg g−1 | 33 | |
| Brazilian bentonite | 0.85 | ||||
| Ni(II) | |||||
| Kaolin | 303 K | 5.11 × 10−5 | mmol g−1 | 27 | |
| Ball clay | 5.62 × 10−3 | ||||
| Kaolinite | 303 K | Ni(II) 10–250 mg L−1, pH 5.7 | 1.1 | mg1−1/n L1/n g−1 | 51 |
| Acid-activated kaolinite | 1.5 | ||||
| Montmorillonite | 4.5 | ||||
| Acid-activated montmorillonite | 6.0 | ||||
| ZrO-kaolinite | 303 K | Ni(II) 10–250 mg L−1, pH 5.7 | 0.8 | mg1−1/n L1/n g−1 | 49 |
| ZrO-montmorillonite | 1.3 | ||||
| Bentonite | 293 K | pH 5.0 | 2.326 | L g−1 | 61 |
Bentonite/iron oxides (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 20 °C) 1 at 20 °C) |
6.730 | ||||
| Montmorillonite K10 | 298 K | 1.000 | mg1−1/n L1/n g−1 | 74 | |
| 3-Mercaptopropyltrimethoxy-silane modified montmorillonite K10 | 0.410 | ||||
| Mg-mesoporous alumina | 298 ± 1 K | Ni(II) 5–30 mg L−1 | 16.62 | mg g−1 | 260 |
| Na-attapulgite | 291.15 K | pH 6.0 ± 0.1 | 0.0026 | mol1−n Ln g−1 | 42 |
| 313.15 K | 0.0011 | ||||
| 333.15 K | 0.0011 | ||||
| Hydrous TiO2 | 288 ± 1 K | Ni(II) 10–150 mg L−1, pH 5.0 ± 1 | 7.69 | mg g−1 | 259 |
| 303 ± 1 K | 8.34 | ||||
| 318 ± 1 K | 11.93 | ||||
| 328 ± 1 K | 13.06 | ||||
| Silico-antimonate ion exchanger | 298 ± 1 K | 0.93 | mmol g−1 | 133 | |
| Dimethylglyoxime treated clinoptilolite | 293 K | 0.138 | L g−1 | 87 | |
| 313 K | 0.128 | ||||
| 333 K | 0.114 | ||||
| Pb(II) | |||||
| Local bentonite | 293.13 | pH 5.2 ± 0.2 | 3.03 × 10−3 | mol1−n Ln g−1 | 32 |
| 323.13 | 5.11 × 10−3 | ||||
| 343.13 | 9.06 × 10−3 | ||||
| Turkish kaolinite | 293 K | pH 6.0 | 0.58 | mg g−1 | 31 |
| Turkish kaolinite | 293 K | pH 5.0 | 0.175 | L g−1 | 30 |
| Kaolin | 303 K | 7.73 × 10−5 | mmol g−1 | 27 | |
| Ball clay | 4.01 × 10−3 | ||||
| Montmorillonite | 298 K | pH 6.0 | 15.06 | L g−1 | 40 |
| Na-bentonite | 298 K | Pb(II) 1.45 × 10−5 to 1.74 × 10−4 mol L−1 | 3.61 × 10−2 | mol1−1/n Ln g−1 | 38 |
| 318 K | 2.57 × 10−2 | ||||
| 338 K | 8.89 × 10−3 | ||||
| Montmorillonite–illite | 310 K | Pb(II) 100 mg L−1 | 9.115 | L g−1 | 251 |
| Pb(II) 150 mg L−1 | 19 150 | ||||
| Pb(II) 200 mg L−1 | 19.600 | ||||
| Acid-activated kaolinite | 303 K | Pb(II) 10–250 mg L−1, pH 5.7 | 0.55 | mg1−1/n L1/n g−1 | 54 |
| Acid-activated montmorillonite | 13.30 | ||||
| Kaolinite | 303 K | 0.808 | mg1−1/n L1n g−1 | 44 | |
| Aluminium sulphate modified kaolinite | 6.94 | ||||
| Kaolinite | 303 K | Pb(II) 10–250 mg L−1, pH 5.7 | 0.37 | mg1−1/n L1/n g−1 | 53 |
| ZrO-kaolinite | 0.34 | ||||
| TBA-kaolinite | 0.31 | ||||
| Montmorillonite | 7.32 | ||||
| ZrO-montmorillonite | 2.01 | ||||
| TBA-montmorillonite | 1.36 | ||||
| 8-Hydroxy quinoline-immobilized bentonite | 58.73 | L g−1 | 71 | ||
| 303 K | 71.84 | ||||
| 313 K | 78.13 | ||||
| 323 K | 91.91 | ||||
| Clay–poly(methoxyethyl)acrylamide composite | 293 K | Pb(II) 100–300 mg L−1 | 3.30 × 10−3 | L g−1 | 75 |
| 303 K | 3.71 × 10−3 | ||||
| 313 K | 3.75 × 10−3 | ||||
| 323 K | 3.23 × 10−3 | ||||
| Natural muscovite | 293 K | Pb(II) 0–100 mg L−1, pH 6.0 | 0.096 | mg1−1/n L1n g−1 | 43 |
| Mordenite | 293 K | Pb(II) 20–100 mg L−1, pH 6.0 | 0.509 | mg1−1/n L1n g−1 | 186 |
| 303 K | 1.080 | ||||
| 313 K | 1.137 | ||||
| 323 K | 1.211 | ||||
| Natural apatite | 0.131 | L mmol−1 | 121 | ||
| Synthetic hydroxyapatite | 0.487 | ||||
| N-Methylimidazole anchored activated palygorskite | 283 K | 51.94 | mg g−1 | 212 | |
| 298 K | 112.17 | ||||
| 313 K | 122.73 | ||||
| Thiol-functionalized silica | 298 K | Pb(II) 20–250 mg L−1 | 63.99 ± 3.92 | L g−1 | 117 |
| Activated alumina | 303 K | pH 5.0 | 4.34 | mg1−1/n L1/n g−1 | 111 |
| Hydrous MnO2 | 298 K | pH 3.2–3.5 | 1.625 | mmol1−n Ln g−1 | 96 |
| NiO | 303 K | Pb(II) 100–600 mg L−1 | 35.46 | mg g−1 | 94 |
| ZnO | 0.152 | mg g−1 | 93 | ||
| Fe3O4 | 298 K | pH 5.5 | 0.128 | mmol1−1/n L1n g−1 | 101 |
| 313 K | 0.145 | ||||
| 328 K | 0.167 | ||||
| TiO2/SiO2 binary mixed oxide | 298 K | pH 6.0 | 1.18 | mol1−1/n L1n g−1 | 98 |
| 308 K | 2.46 | ||||
| 325 K | 0.13 | ||||
| Mg2Al LDH | 303.15 K | Pb(II) 5–23 mg L−1, pH 5.7 ± 0.01 | 19.76 | mg g−1 | 119 |
| 323.15 K | 23.31 | ||||
| 343.15 K | 30.91 | ||||
| River sand | 298 ± 2 K | 15.4 ± 6.9 | mmol g−1 | 125 | |
| Sand | 293 K | 0.28 ± 0.19 | mmol g−1 | 126 | |
| 303 K | 0.63 ± 0.09 | ||||
| 313 K | 5.30 ± 3.61 | ||||
| 323 K | 5.10 ± 1.28 | ||||
| Iron oxide-immobilized sand | 298 ± 1 K | 0.785 | mg g−1 | 128 | |
| Loamy sand soil | 387.1 | mg1−1/n L1/n kg−1 | 124 | ||
| Titanate nanotube | 298 ± 1 K | Pb(II) 0.1–3.0 mmol L−1 | 1.22 | mmol g−1 | 80 |
| Electric furnace slag | 293 K | 0.57 | mg1−1/n L1n g−1 | 137 | |
| 303 K | 0.93 | ||||
| 313 K | 1.82 | ||||
| Dithiocarbamate chelating resin | 298 K | Pb(II) 1–25 mmol L−1 | 0.588 | mmol L−1 | 136 |
| Zn(II) | |||||
| Kaolin | 303 K | 1.07 × 10−3 | mmol g−1 | 27 | |
| Ball clay | 5.32 × 10−3 | ||||
| Bentonite | 293 K | pH 3.0 | 1.100 | mg g−1 | 34 |
| pH 5.0 | 1.732 | ||||
| pH 7.0 | 10.063 | ||||
| pH 9.0 | 12.320 | ||||
| HCl/NaOH-treated bentonite | 295 ± 1 K | Zn(II) 20–200 mg L−1 | 71.22 | mg g−1 | 45 |
| Hydrous MnO2 | 298 K | pH 3.5–4.0 | 1.002 | mmol1−n Ln g−1 | 96 |
| Silico-antimonate ion exchanger | 298 ± 1 K | 0.47 | mmol g−1 | 133 | |
(i) Kaolin: 6.22 × 10−4, 1.13 × 10−3, 4.20 × 10−4, 5.11 × 10−5, 7.73 × 10−5 and 1.07 × 10−3 mmol g−1.
(ii) Ballclay: 3.11 × 10−3, 2.31 × 10−2, 7.16 × 10−3, 5.62 × 10−3, 4.01 × 10−3 and 5.32 × 10−3 mmol g−1 respectively for Cd(II), Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II). The ballclay having 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layer structure has higher charges in both tetrahedral and octahedral sheets resulting from isomorphous substitution, whereas kaolin (1
1 layer structure has higher charges in both tetrahedral and octahedral sheets resulting from isomorphous substitution, whereas kaolin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) has little such substitution. This explains the higher adsorption capacity of ball clay. The highest adsorption affinity of Cr(III) on both the clays was attributed to its small ionic radius and higher ionic charge. For the divalent metal ions, a broad relationship between relative affinity and the tendency to hydrolyze was shown to determine their selectivity sequence. The results are also in general agreement with the CEC of 11.3 mmol per 100 g for kaolin and 14.9 mmol per 100 g for ballclay as reported by the authors.
1) has little such substitution. This explains the higher adsorption capacity of ball clay. The highest adsorption affinity of Cr(III) on both the clays was attributed to its small ionic radius and higher ionic charge. For the divalent metal ions, a broad relationship between relative affinity and the tendency to hydrolyze was shown to determine their selectivity sequence. The results are also in general agreement with the CEC of 11.3 mmol per 100 g for kaolin and 14.9 mmol per 100 g for ballclay as reported by the authors.
The adsorption process of Cr(III) on kaolinite was influenced by solution pH, ionic strength and temperature.28 The kaolinite surface in water had a net negative surface charge at natural pH with an isoelectric point at ∼pH 2.35. Similar work was reported by Arias and Sen29 for Zn(II) removal by kaolin with KF of 1.61 mg g−1. Sari et al.30 proposed that Pb(II) ions from water were held by ionisable surface groups located at the edges of Turkish kaolinite and had KF of 0.175 L g−1 at 293 K.
A local clay material consisting of 59.46% SiO2 and 14.92% Al2O3 had KF of 0.54 and 0.58 mg g−1 respectively for Cr(III) and Pb(II).31
The maximum KF for Pb(II) on a local bentonite32 was 9.06 × 10−3 mol1−n Ln g−1 at 343.15 K and it was suggested that pH of the medium determined whether inner-sphere complexation or chemisorption was the mechanism by which Pb(II) was held to Na-bentonite.
Natural Brazilian bentonite (NT-25) had much larger Freundlich capacity33 than the commercial montmorillonite, K10 (NT-25: Cd(II) 1.74 mg g−1; Mn(II) 0.85 mg g−1; K10: Cd(II) 0.42 mg g−1; Mn(II) 0.31 mg g−1). Hard Lewis acids and bases tend to form outer-sphere complexes and their adsorption capacity occurs mostly in surfaces with an excess of negative charge. Brazilian bentonite was also shown to have a larger CEC (74.8 mmol per 100 g) compared to K10 montmorillonite (45.1 mmol per 100 g).
Natural bentonite had KF of 0.46 to 3.83 mg g−1, 1.02 to 8.48 mg g−1 and 1.10 to 12.32 mg g−1 in the pH range of 3.0 to 9.0 respectively for Co(II), Cu(II) and Zn(II).34 The surface charge density turns more negative at high pH taking up more of the cations. The CEC of bentonite was shown to be pH dependent with values of 45.50 mmol per 100 g (pH 5.0), 51.85 mmol per 100 g (pH 6.35) and 62.40 mmol per 100 g (pH 8.0). The adsorption capacity is attributed to ion-exchange reactions in the micropores of the mineral at comparatively high pH. This was also the case with Zn(II) adsorption on bentonite35 with Freundlich capacity of 1.46 mg g−1 at 303 K. In another work on bentonite clay, Karapinar and Donat36 found the Freundlich capacities for Cd(II) and Cu(II) to be 46.56 and 19.54 mg g−1 respectively.
Freundlich capacity of Na-bentonite for Cu(II)37 varied from 5.77 × 10−4 to 5.91 × 10−4 mol1−n Ln g−1 in the temperature range, 293.15 to 333.15 K and Cu(II) ions were held through ion exchange with H+/Na+ of the surface at low pH. At pH > 6.5, Cu(II) species present are Cu(OH)2, Cu(OH)+ and Cu(OH)22+ that bind to negatively charged sites of Na-bentonite surface. It was also found that Na-bentonite had a higher Freundlich capacity for Pb(II) (KF: 3.61 × 10−2 to 8.89 × 10−3 mol1−n Ln g−1 in the temperature range, 298 to 338 K) than raw bentonite.38 These differences were due to favourable properties of higher cation exchange capacity (91 mmol per 100 g), better dispersibility and better thermostability. Pb(II) adsorption on Na-bentonite was dominated by ion exchange or outer-sphere surface complexation at low pH, and by inner-sphere surface complexation at high pH.
Natural bentonite–Zn(II) interactions had higher Freundlich capacity than bentonite–Cu(II) (Zn(II): 16.22 mg g−1, Cu(II) 8.71 mg g−1).39 While exchangeable cations present in bentonite surface participated in ion-exchange type adsorption, higher ionic potential of Zn(II) compared to Cd(II), must have helped it to bind strongly with the clay surface.
Zhang and Hou40 suggested that adsorption capacity of montmorillonite (CEC 73.16 mmol per 100 g) for Pb(II) (KF: 15.06 L g−1) arose partly from chemical binding occurring at surface hydroxyl groups and partly from electrostatic interactions with the permanent negatively charged sites on montmorillonite.
Thermal or chemical treatments were also used to enhance adsorption capacity of clay. Thus, the material obtained by treatment (900 °C + sodium acetate + acetic acid + sodium nitrate) of a local clay had better adsorption capacity for Cd(II), Pb(II) and Zn(II).41
Strong surface complexation was suggested to be responsible for adsorption of Ni(II) on Na-attapulgite with KF varying from 2.6 × 10−3 to 1× 10−3 mol1−n Ln g−1 in the temperature range of 291.15 to 333.15 K.42
Natural muscovite43 adsorbed As(III), As(V), Cd(II), Cu(II) and Pb(II) with Freundlich capacities of 0.03, 0.20, 0.01, 0.03 and 0.10 mg1−1/n L1/n g−1 respectively at 293 K. The overall adsorption capacity was influenced by impurities such as quartz and undetermined minerals present on the natural muscovite. Muscovite has a low surface area and a very low CEC (0.28 mmol per 100 g) compared to most other natural minerals explaining its low adsorption capacities.
When bentonite (CEC 51.20 mmol per 100 g) was modified by treating with HCl and subsequently with NaOH, Freundlich capacities for Co(II) and Zn(II) were 1.02 and 71.22 mg L−1 respectively.45 The modification, according to the authors, opened up more macro and micro pores in the clay mineral that helped in removing more metal ions.
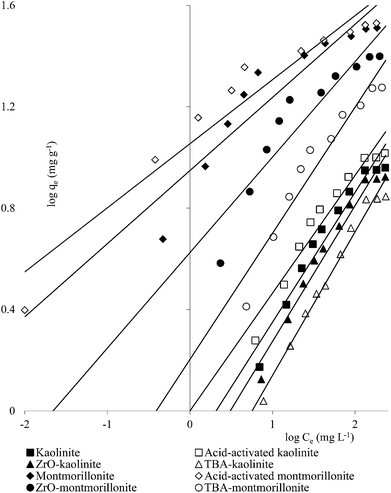
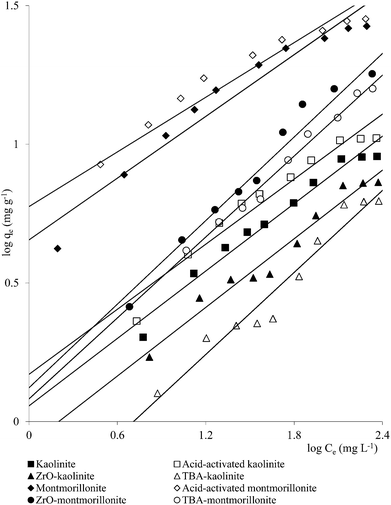
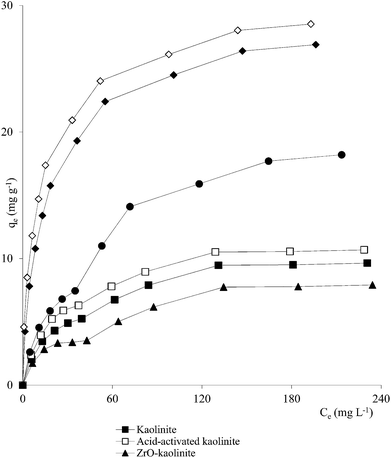
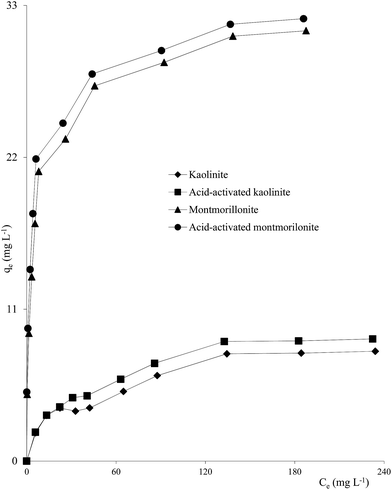
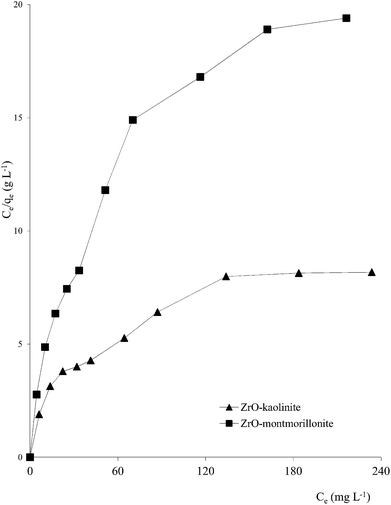
Clay mineral based adsorbents (kaolinite, montmorillonite, ZrO-kaolinite, ZrO-montmorillonite, acid-activated kaolinite and acid-activated montmorillonite) were shown to have excellent adsorption properties for Cd(II), Co(II), Cu(II), Cr(VI), Fe(III), Ni(II), and Pb(II).46–57 The montmorillonite-based adsorbents possessed higher adsorption capacity (Cd(II): 1.2 to 12.9, Co(II): 1.1 to 6.0, Cu(II): 1.1 to 12.4, Cr(VI): 1.0 to 1.5, Fe(III): 1.3 to 6.4, Ni(II): 1.1 to 6.0, Pb(II): 0.4 to 13.3 mg1−1/n L1/n g−1) (Fig. 1–6). The raw montmorillonite was almost five times as effective in adsorbing metal ions as kaolinite. The introduction of Zr(hydr)oxide did not improve the adsorption capacity of either montmorillonite or kaolinite. The poly(hydroxo zirconium) ions might have masked the negatively charged sites and blocked the pores. Acid activation, however, enhanced the adsorption capacity of the clay minerals through increase in surface area as well as pore volume. The results were in conformity with the CEC values measured by the authors (kaolinite: 11.3 mmol per 100 g, ZrO-kaolinite: 10.2 mmol per 100 g, acid-activated kaolinite: 22.0 mmol per 100 g) compared to their montmorillonite counterparts (montmorillonite: 153.0 mmol per 100 g, ZrO-montmorillonite: 73.2 mmol per 100 g, acid-activated montmorillonite: 341.0 mmol per 100 g).
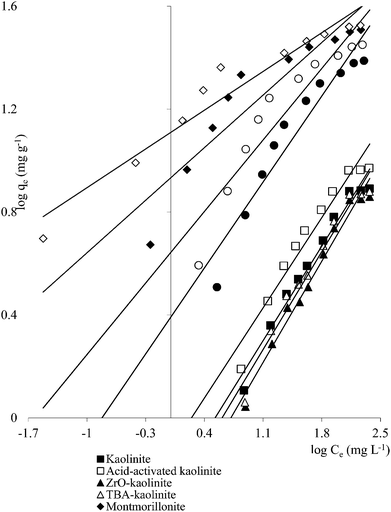 | ||
| Fig. 1 Freundlich plots for Cd(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Cd(II) 10–250 mg L−1, pH 5.5, time 240 min, temperature 303 K). | ||
 | ||
| Fig. 2 Freundlich plots for Pb(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Pb(II) 10–250 mg L−1, pH 5.7, time 180 min, temperature 303 K). | ||
 | ||
| Fig. 3 Freundlich plots for Ni(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Ni(II) 10–250 mg L−1, pH 5.7, time 180 min, temperature 303 K). | ||
 | ||
| Fig. 4 Plots of qe vs. Ce for Co(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Co(II) 10–250 mg L−1, pH 5.8, time 240 min, temperature 303 K). | ||
 | ||
| Fig. 5 Plots of qe vs. Ce for Cu(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Cu(II) 10–250 mg L−1, pH 5.7, time 360 min, temperature 303 K). | ||
 | ||
| Fig. 6 Plots of qe vs. Ce for Fe(III) adsorption on clay minerals (clay mineral 2.0 g L−1, Fe(III) 10–250 mg L−1, pH 3.0, time 300 min, temperature 303 K). | ||
Adsorption of Cd(II) and Cu(II) at 303 and 323 K on natural and modified bentonite (B) [modified with goethite (G), humic acid (H) and binary mixture of goethite/humic acid (G + H)]58 showed that the humic acid modified species had the highest Freundlich capacity [for e.g., at 323 K, KF for Cd–B + H: 2.82, Cd–B + G + H: 2.65, Cd–B + G: 1.08, Cd–B: 2.40 mg g−1; KF for Cu–B + H: 3.13, Cu–B + G + H: 2.11, Cu–B + G: 2.76, Cu–B: 2.87 mg g−1]. Modification with either goethite or humic acid might have introduced some new sites onto bentonite. Humic acid created strong complexing sites on bentonite that could take up more metal ions. These results were reflected in the increased CEC values [B: 95 mmol per 100 g, B + G: 105.32 mmol per 100 g, B + H: 120.4 mmol per 100 g, B + G + H: 125.8 mmol per 100 g].
KF (8.93 ± 0.90 mg1−1/n L1/n g−1) of MgO-coated bentonite for Cu(II) removal was much higher than that of the raw clay.59 The higher adsorption was suggested to be due to the coordinative environments of Cu(II) ions and surface hydroxyl groups in a hydrated surface. MgO could have acted as a stabilizing buffer, which minimized metal solubility and avoided redissolution. Mixed Fe, Mg (hydr)oxides coated bentonite composite had Freundlich capacity of 35.19 mg1−1/n L3/n g−1 for Pb(II) with ion-exchange of Mg(II) by Pb(II) being the dominant process.60
Freundlich capacities for natural bentonite and bentonite/iron oxide composite of Ni(II)61 were 2.33 and 6.73 L g−1 respectively at 293 K. The increased specific surface area and pore volume of composites compared to the natural bentonite suggested formation of agglomerated structures from precipitated nanosized iron oxides on adsorbent surface responsible for higher removal of Ni(II).
Fe–Ni bimetallic-montmorillonite nanocomposites possessed higher Freundlich adsorption capacity (69.13 mg g−1) compared to zero-valent iron (56.88 mg g−1) and Fe–Ni bimetallic nanoparticles (64.71 mg g−1) for removal of Cr(VI).62 Adsorption and reduction of Cr(VI) to Cr(III) occurred simultaneously and the authors suggested existence of a facile electron transfer from nanoparticle to the adsorbed Cr(VI) species resulting in faster reduction. Strong ionic interactions between Cr(VI) and composite surface were shown to occur through pore diffusion enhancing adsorption–desorption dynamics.
Use of different metal cations for pillaring on clays influences the Freundlich adsorption capacity. Tomul63 has shown that Cd(II) adsorption on Fe pillared bentonite (0.451 L g−1), Fe/Cr pillared bentonite (0.347 L g−1) and Cr pillared bentonite (0.264 L g−1) had different adsorption capacities at 298 K. Since pore structure and surface properties of an adsorbent have a significant effect on the adsorption process, and the distribution of pores affects the efficiency and selectivity of adsorption, Fe pillared bentonite, Fe/Cr pillared bentonite and Cr pillared bentonite differ in their adsorption capacities due to differences in pore distribution.
Yan et al.64 have shown that the Freundlich adsorption capacities of (i) acid-activated Al13-pillared Na+-montmorillonite (Al13-PAAMt), (ii) Al13-pillared Na+-montmorillonite (Al13-PMt) and (iii) Na+-montmorillonite (Na+-Mt) with respect to Cd(II) were in the order of (i) > (ii) > (iii). The uptake of Cd(II) was influenced by the changes in basal spacing, specific surface area and total pore volume due to intercalation with inorganic polyoxycations and also acid treatment.
In a significant earlier work, Jobstmann and Singh26 found the Freundlich adsorption capacities of natural montmorillonite (3775.5 μM1−n kg−1 Ln) for Cd(II) to be almost one order higher than that of hydroxy-aluminium-interlayered-montmorillonite (507.0 μM1−n kg−1 Ln). The pillared clay had n < 1 suggesting the presence of energetically non-uniform sites while the unpillared montmorillonite had n close to 1 (0.85) that showed that the surface could be considered as nearly homogeneous. According to the authors, Cd(II) occupied both permanent charge sites and broken bond AlOH sites and the higher adsorption capacity of the natural montmorillonite was due to Cd(II) being more effectively adsorbed at the interlayer sites rather than inside the pores. Moreover, the CEC of pillared montmorillonite (64.5 mmol per 100 g) was ∼33% lower than the unpillared montmorillonite (96.6 mmol per 100 g).
Fe-montmorillonite had almost 4 times as large Freundlich capacity for Cd(II) (KF: 16.9 L g−1) than that of Ca-montmorillonite (parent clay, KF: 3.90 L g−1).65 The higher value was correlated to large interlayer spacing in Fe-montmorillonite. Even when humic acid was used to modify Ca-montmorillonite, Freundlich adsorption capacity for Cd(II), Cu(II) and Cr(III) was higher than that of the raw clay, but the increase was now around 25% only.66 Humic acid introduced multiple functional groups to the clay mineral while at the same time blocking some of the pores and therefore, the increase in adsorption capacity was much below the expected value.
The preferential removal of As(V) over As(III) was observed by Su et al.68 with octadecyl benzyl dimethyl ammonium-modified bentonite as the adsorbent when the Freundlich capacity for As(V) was more than 4 times that for As(III). At pH < 9.0, the predominant form of As(III) in aqueous solution is H3AsO3 such that anion exchange could not be the mechanism for As(III) removal. Whatever As(III) is removed is through a physical adsorption process involving Si–O and Al–O groups at the edges of the particles.
A number of metal ions [Cd(II), Cr(VI), Ni(II), Pb(II)] was adsorbed on the clay minerals, kaolinite and montmorillonite, as well as on tetrabutylammonium (TBA)-kaolinite and TBA-montmorillonite47,53,55,69 with the Freundlich capacities in a broad range of values (Cd(II): 0.32 to 2.21, Cr(VI): 0.9 to 1.1, Ni(II): 3.4 to 1.13, Pb(II): 0.37 to 1.36 mg1−1/n L1/n g−1). As already pointed out, introduction of TBA might have blocked the pores leaving no scope for increasing the adsorption capacities (Fig. 1–3). The modified clays had lower CEC than the respective parent clays (kaolinite: 11.3 mmol per 100 g, TBA-kaolinite: mmol per 100 g, montmorillonite: 153.0 mmol per 100 g, TBA-montmorillonite: mmol per 100 g).
Bentonite with 8-hydroxy quinoline immobilized on its surface had Freundlich capacity for Cu(II)70 of 34.78 to 41.16 L g−1 and for Pb(II)71 of 58.73 to 91.91 L g−1 in the temperature range, 293 to 323 K. The FTIR intensities of C–C and C–N ring stretching vibrations were shown to increase after adsorption of Cu(II) or Pb(II). Another material obtained by immobilizing 2,2′-dipyridyl on bentonite gave KF for Cu(II) adsorption of 11.20 to 30.02 L g−1 in the temperature interval, 293 to 323 K (n 0.127 to 0.281).72 C–C and C–N ring stretching (skeletal) FTIR bands were observed after Cu(II) adsorption.
Dodecylamine modified sodium montmorillonite yielded a Cr(VI) Freundlich capacity73 of 3.27 mg1−1/n g−1 L1/n. The negatively charged hydrogentetraoxochromate (HCrO4−) and the protonated dodecylamine could have interacted via an electrostatic force of attraction in the clay matrix.
Carvalho et al.74 reported Freundlich capacities for adsorption of Ni(II) from water on montmorillonite K10 and modified montmorillonite K10 (modified with 3-mercaptopropyltrimethoxysilane) as 1.00 and 0.41 mg1−1/n L1/n g−1 respectively at 298 K.
Şölener et al.75 measured KF of clay–poly(methoxyethyl)acrylamide composite for Pb(II) from 3.30 × 10−3 L g−1 (293 K) to 3.75 × 10−3 L g−1 (313 K) which decreased to 3.23 × 10−3 L g−1 (at 323 K). The interactions occurred through olefinic double bond, carbonyl and amine groups, etc.
In adsorption of Cr(VI) on tannin-immobilized activated clay,76 KF increased from 2.41 to 3.11 L g−1 when the solution temperature was increased from 300 to 330 K. It was suggested that Cr(VI) anions were partly reduced to Cr(III) which were adsorbed as cations.
Adsorption capacity of sepiolite was boosted after acid activation and surface functionalization with mercaptosilane with respect to Cr(VI) uptake.77 The modified sepiolite had higher content of silanol groups, which resulted in a higher adsorption capacity. The same group of workers78 reported Cr(VI) adsorption from water on natural and acid-activated sepiolites, functionalized by covalent grafting [3-(2-aminoethylamino)propyl]trimethoxy-silane from water. The Freundlich capacities were 2.042 and 2.845 mg1−1/n g−1 L1/n respectively. The electrostatic attraction of anionic Cr(VI) species by protonated amine groups and the formation of hydrogen bonds between the CrO42− species and the non-protonated amine groups were possible mechanisms of metal ion removal at pH 2.0. Adsorption of Pb(II) on mercapto functionalized sepiolite was facilitated by the formation of bidentate complexation with mercapto groups besides monodentate complexation, as suggested by Liang et al.79 The increase of reaction temperature from 289.15 to 318.15 K boosted the Freundlich capacity from 21.89 ± 1.97 to 33.73 ± 1.28 mg g−1.
Freundlich capacity of natural zeolite (clinoptilolite) for Fe(III) was higher (0.376 mg g−1) compared to Zn(II) (0.015 mg g−1) and Mn(II) (0.035 mg g−1).81 The surface charge imbalance created by substitution of Si4+ by Al3+ was balanced by exchangeable cations. Similarly, natural zeolitic volcanic tuff yielded Freundlich capacities of 2.88, 2.50 and 2.07 mg g−1 for 293, 313 and 333 K respectively for Pb(II) adsorption.82
In another work, natural zeolite showed higher Freundlich capacity for Pb(II) (KF: 59.56 L mg−1) than for Cu(II) (KF: 9.08 L mg−1) at 303 K.83
NaA and NaX zeolites contain mineral impurities in the form of Ca, Mg, Fe and K and when these materials are used as adsorbents for Zn(II), the impurities interfere in the exchange of Zn(II) with Na(I).84 NaA and NaX have small particles size and higher internal surface area allowing Zn(II) cations to access and diffuse into the framework structure. This has resulted in high KF of 85.54 and 77.24 L g−1 respectively for NaA and NaX.
Argun85 reported decrease in KF of clinoptilolite for Ni(II) adsorption from 0.68 to 0.53 mg g−1 when the temperature changed from 293 to 333 K.
Aluminium-loaded Shirasu-zeolite (Al-SZP) had a reasonable Freundlich capacity of 0.122 mmol g−1 for As(V) and adsorption intensity of 0.13.86 The adsorption capacity of the material was better than activated alumina. The adsorption of As(V) onto Al-SZP proceeded from the formation of aluminum hydroxide on the surface, followed by the replacement of the hydroxide anion by As(V) in aqueous media.
Ni(II) adsorption by both ion-exchange and complexation processes on dimethylglyoxime treated clinoptilolite yielded Freundlich capacities in the range of 0.138 to 0.114 L g−1 (temperature 293 to 333 K).87
Adsorption of Cu(II) on humic acid immobilized-cetylpyridinium bromide-modified zeolite88 followed ion exchange in the internal zeolite channels.
Freundlich capacity for Cd(II) removal on zeolite-based geopolymer (synthesized from coal fly ash)89 was 6.603 L g−1. The adsorbent was fully coated with hydroxyl compounds that penetrated into the porous network structure that played a role in the adsorption process.
Three different minerals, vermiculite, zeolite and pumice were used for adsorption of Cd(II) from water.90 KF had values of 0.69, 0.65 and 0.55 L g−1 for vermiculite, zeolite, and pumice respectively. Increasing amount of adsorption was shown to modify the adsorbent characteristics making chemical interactions as the dominant process. Zeolite and vermiculite adsorbed Cd(II) to a larger extent compared to pumice in line with the CEC values of the materials (CEC 170 mmol per 100 g, 100 mmol per 100 g and 30 mmol per 100 g for zeolite, vermiculite and pumice, respectively) and the adsorption percentage was higher at the lowest Cd(II) concentration.
In a comparative study, Sheela et al.92 found that nano ZnO had Freundlich capacity in the order of Zn(II) (KF: 2.234 mg g−1), Cd(II) (11.77 mg g−1) and Hg(II) (17.32 mg g−1). It was suggested that, during the possible ion exchange process, metal cations moved through either the pores of ZnO mass or through channels of the crystal lattice. Moreover they might replace exchangeable cations, mainly surface hydroxyl groups. The interaction between nano ZnO and Pb(II) yielded Freundlich capacity of 0.152 mg g−1, the larger surface area of the adsorbent might play some role in the uptake process.93
NiO nanoparticles had a preferential uptake of Pb(II) (KF: 34.45 mg g−1) over Cd(II) (KF: 6.61 mg g−1) attributed to lower pH of hydrolysis of Pb(II).94
With Al2O3 as the adsorbent,95 Cd(II) cations were shown to bind to the surface through specific adsorption on (Al–OH), resulting in the formation of monodenate or bidenate complexes with Al–O−. Freundlich capacities for Cd(II), Pb(II) and Zn(II) adsorption on hydrous MnO2 were 1.38, 1.63 and 1.00 mmol1−n Ln g−1 respectively at 298 K.96 An ion-exchange mechanism was proposed in which release of H+ was accompanied by Cd(II) and Pb(II) uptake.
Chen et al.97 found that the Freundlich capacities of Cd(II) adsorption on nano TiO2 and humic acid-modified TiO2 were influenced by solution pH [pH 5.0: KF (5.22 ± 0.8) × 10−2 μg1−1/n L1/n g−1 for TiO2 and (5.73 ± 0.8) × 10−2 μg1−1/n L1/n g−1 for HA–TiO2; pH 7.0: (7.31 ± 2.5) × 10−2 μg1−1/n L1/n g−1 and (5.33 ± 2.5) × 10−2 μg1−1/n L1/n g−1 for TiO2 and HA–TiO2 respectively and at pH 9.0: (4.09 ± 0.6) × 10−2 μg1−1/n L1/n g−1 and (9.90 ± 2.77) × 10−2 μg1−1/n L1/n g−1 for TiO2 and HA–TiO2 respectively]. The humic acid modification increased the adsorption capacity due to smaller aggregation sizes and better dispersion of nano particles in the presence of HA. Aliphatic chains constitute the basic structure for HA fractions on modified TiO2, and they might form net, stretch, or spongy structures below pH 7.0, but condensed at pH > 7.0. Therefore, with increasing pH, the number of available adsorption sites for Cd(II) increase. The adsorption ability of modified TiO2 was stronger than that of the unmodified TiO2 in weak acid and weak base solutions. It was suggested that the surface charges of HA–TiO2 might change quickly than those of TiO2 at pH 7 giving a lower Freundlich capacity for HA–TiO2 than TiO2.
Pb(II) adsorbed on nano TiO2–SiO2 with Freundlich capacities of 1.18 to 2.46 mol1−1/n L1/n g−1 in the temperature range of 298 to 308 K and decreased to 0.13 mol1−1/n L1/n g−1 at 325 K.98 It was proposed that Pb(II) bonded with TiO2–SiO2 surface via surface oxygen atoms and proton release.
Liu et al.99 used titanate nanotubes for removal of a few metal ions with Freundlich capacities of 1.22 mmol g−1 (Pb(II)), 0.92 mmol g−1 (Cd(II)), 0.88 mmol g−1 (Cu(II)) and 0.87 mmol g−1 (Cr(III)). It was suggested that adsorption of metal ions by the adsorbent could be possible by –OH and –ONa located in titanate interlaminations. The adsorption mechanism followed ion-exchange between H+/Na+ and metal ions.
Freundlich capacity for Se(IV) adsorption on iron oxide was more than that on silicon oxide.100 KF decreased from 1.09 to 0.34 mg1−1/n L1/n mg−1 and 0.90 to 0.46 mg1−1/n L1/n mg−1 for iron oxide and silicon oxide respectively (at 303 to 323 K). Se(IV) binding to the oxides were through electrostatic and inner-sphere complexation. Several inner-sphere complexes could be formed at the surface of ferric oxide: monodentate (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) S–O–Se(O)OH,
S–O–Se(O)OH, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) S–O–Se(O)O− or
S–O–Se(O)O− or ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) S–O–Se(O−)2OH), bidentate (
S–O–Se(O−)2OH), bidentate (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) S–O)2–SeO and tridentate (
S–O)2–SeO and tridentate (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) S–O)3–SeOH.
S–O)3–SeOH.
Freundlich capacity of Fe3O4 nanoadsorbents for Pb(II) removal101 was in the range of 0.13 to 0.17 mmol1−1/n L1/n mol−1 with increasing temperature (298 to 328 K) and endothermic interactions. As(V) anions as H2AsO4− were shown to have bound to Fe3O4 nanoparticles on positive charge sites of the surface yielding a Freundlich capacity of 1.238 μg1−1/n g−1/n L1/n.102
Al2O3-supported iron oxide yielded Freundlich capacity of 6.15 to 11.15 for Pb(II) in the temperature range, 288 to 318 K.103 The solid surface was shown to contain α-FeOOH that bound Pb(II).
Bimetallic nano-oxides, such as MnFe2O4 and CoFe2O4, had Freundlich adsorption capacities almost twice that of Fe3O4 for As(III) (MnFe2O4–As(III): 29.6 mg1−1/n L1/n g−1, CoFe2O4–As(III): 36.9 mg1−1/n L1/n g−1, Fe3O4–As(III): 15.9 mg1−1/n L1/n g−1).104 The O1s binding energies from XPS measurements were used to suggest that higher adsorption capacities of the bimetallic oxides for As(III) and As(V) compared to Fe3O4 were due to increase in the number of surface hydroxyl species.
Zhang et al.,105 while using Ce doped Fe-oxide as an adsorbent for As(V), considered the surface as amphoteric with the presence of surface hydroxyl groups (M–OH). Higher As(V) removal at pH > 5.8 was suggested as due to specific adsorption of As(V) anions. The same group of authors106 latter prepared a series of nanosized hydrated ferric oxide (HFO)-loaded polymeric hybrid adsorbents and showed that Freundlich adsorption capacity for As(V) was mainly dependent upon HFO loadings. Adsorbents with low HFO loadings exhibited better performance at relatively low solute levels, while those with higher HFO loadings displayed higher adsorption capacities at higher solute levels. However, large HFO loadings resulted in a dramatic decrease in As(VI) adsorption arising from blocking of the pores and difficult accessibility of the active sites.
Fe3O4 nanoparticles modified with 3-aminopropyltriethoxysilane and glutaraldehyde adsorbed Cu(II) from aqueous system107 with Freundlich capacity of 14.544 mmol1−1/n L1/n g−1.
Fe3O4 modified with cetyltrimethylammonium bromide was shown to possess higher uptake capacity for As(V) compared to pure Fe3O4.108 CTA+ present on the surface of Fe3O4 held As(V) anions forming CTA–As(V) complex.
Carboxymethyl-β-cyclodextrin polymer modified Fe3O4 nanoparticles had Freundlich capacities of 13.44 to 25.82 L g−1 for Pb(II), 0.426 to 17.64 L g−1 for Cd(II) and 0.755 to 2.39 L g−1 for Ni(II) at 298 K.109 The polymer grafted on nanoparticles enhanced the adsorption capacity because of the complexing abilities of the multiple –OH and –COOH groups in polymer with metal cations. In a similar work, Tan et al.110 used amino-functionalized Fe3O4 magnetic nano-particles for Pb(II) removal with Freundlich capacity 21.9 L g−1.
Freundlich adsorption capacity for Cd(II) and Pb(II) on activated alumina was 4.34 and 3.82 mg1−1/n L1/n g−1 respectively at 303 K.111 A mechanism based on ion exchange inside the micropores was proposed in which the metal ions entered the pores and also the channels of the lattice where they replaced the exchangeable cations. Pore diffusion was fast and was retarded only when the cations had to move through the smaller channels.
Freundlich capacity was slightly greater for protonated mesoporous alumina compared to aminated alumina for Cu(II) while the reverse was true in case of adsorption intensity (0.48 and 0.53) at 298 K.112 Cu(II) ions were exchanged for aminated and protonated groups of compounds present in the material.
Large surface area of goethite ensured comparatively large Freundlich capacities of 2.140 and 2.227 L g−1, respectively for adsorption of Cd(II) and Cu(II).113
Granados-Correa et al.114 measured Cd(II) KF of 0.11 and 0.07 mg1−1/n L1/n g−1 respectively for bohemite and goethite. Bohemite has higher surface area and larger number of functional groups than goethite, which explains the higher uptake. Cd(II) was proposed as having directed chemical bond to the surface, implying a specific adsorption mechanism.
Manu et al.115 used three different functionalized silica samples (prepared with 3-aminopropyltrimethoxysilane (APTMS)) with different APTMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica ratio (w/w) to adsorb Cu(II) from water. KF increased from 1.416 to 17.132 mg1−1/n L1/n g−1 with increasing APTMA
silica ratio (w/w) to adsorb Cu(II) from water. KF increased from 1.416 to 17.132 mg1−1/n L1/n g−1 with increasing APTMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica ratio. Increase in the concentration of amino groups on the surface of silica resulted in an increase in affinity for Cu(II).
silica ratio. Increase in the concentration of amino groups on the surface of silica resulted in an increase in affinity for Cu(II).
Freundlich adsorption capacity on amino functionalized mesoporous silica (NH2-MCM-41) followed the order Cd(II) > Ni(II) > Pb(II) at 298 K.116 Introduction of amine groups into MCM-41 gave much higher adsorption capacity for Ni(II) and Cd(II). The metal cations were bound to the surface via N-atoms of primary amine groups through complex formation. In a similar work, it was found that thiol-functionalized silica adsorbed Cd(II) and Pb(II) with Freundlich capacity of 25.65 ± 1.43 and 63.99 ± 3.92 L mg−1 respectively at 298 K.117 In this case, cation binding involved two or more –SH groups. Both monodentate and bidentate complexation with thiol groups was proposed.
Surface –OH groups in Mg2Al layered double hydroxides were responsible for large KF of 19.76 to 30.91 mg1−1/n Ln g−1 for Pb(II) in the temperature range of 303.15 to 343.15 K.119
Nano hydroxyapatite had a large Freundlich capacity for Pb(II) (282.308 mg g−1) compared to that for Cd(II) (122.854 mg g−1) and Ni(II) (9.806 mg g−1).120 The preferred adsorption of Pb(II) was justified on the basis of smaller hydrated radius of Pb(II). Synthetic hydroxyapatite also had a large Pb(II) Freundlich capacity (KF: 0.487 L mmol−1) compared to that of natural apatite (KF: 0.131 L mmol−1).121
Tourmaline (a natural hydrous silicate of CEC, 384 mmol per 100 g) was shown to bind Pb(II) through surface electropolar forces122 arising from electric dipoles on the surface and yielded KF of 0.53 L mg−1.
Wang et al.123 showed that KF values of loess soil for Cd(II) increased from 3.80 to 4.87 mg g−1 in the temperature range, 278 to 318 K and Cd(II) ions were held to soil surface by formation of CdSiO3 with aluminium silicate minerals. The Freundlich intensity factor, n, was very small (n = 0.14 to 0.20) indicating largely heterogeneous energy distribution in the soil surface. The CEC of the loess soil was 11.2 mmol per 100 g, but influence of temperature on CEC was not indicated.
Cr(VI) and Pb(II) adsorption was suggested to have occurred through hydroxyl groups on the surface and edges of soil particles on a loamy sandy soil with Freundlich capacity of 1078.0 and 387.1 L1/n mg kg−1 mg−1/n respectively.124
Ahmed et al.125 used river sand for Pb(II) adsorption with Freundlich capacity and intensity of 15.4 ± 6.9 mmol g−1 and 0.71 ± 0.01, respectively (adsorbent 100 mg, stirring speed 700 rpm, temperature 298 ± 2 K). A similar investigation was reported where beach sand was used as adsorbent for Pb(II)126 with KF of 0.28 ± 0.19 mmol g−1 (n= 0.39 ± 0.09) to 5.10 ± 1.28 mmol g−1 (n= 0.67 ± 0.06) at 293 to 323 K. Beach sand was also used for Zn(II) removal (KF: 0.49 ± 0.05 mmol g−1).127 The sand contained calcite, quartz and aragonite as the main constituents. The presence of negative sites in these minerals in the form of carbonates and oxides provided affinity for the positively charged metal ions in aqueous solutions. Iron-oxide nano-particle-immobilized sand was used for removal of Cd(II), Cu(II) and Pb(II) with Freundlich capacities of 0.18, 0.49 and 0.78 mg g−1 respectively.128 It was suggested that Cu(II) adsorption followed ion-exchange as well as inner-sphere surface complexation, while Cd(II) and Pb(II) were held through ion-exchange and electrostatic attraction. Exothermic adsorption of Cr(VI) on acid activated river sand had Freundlich capacity of 0.17 to 0.10 mg g−1 in the temperature range of 298 to 308 K.129
Hydroxyapatite–chitosan composite preferentially adsorbed Pb(II) (4.039 mg1−1/n L1/n g−1) compared to Co(II) (2.619 mg1−1/n L1/n g−1) and Ni(II) (1.577 mg1−1/n L1/n g−1). The presence of amino and hydroxyl groups in chitosan served as the active sites for adsorption.130
Chitosan–clay–magnetite composites were used for Cu(II) and As(V) removal131 with higher uptake for Cu(II) (KF for Cu(II): 5.828 L g−1 and As(V): 0.282 L g−1). The clay had a large surface area for incorporating nano-magnetite and chitosan while preventing particle agglomeration, and therefore, had better adsorption capacity.
Chitosan–attapulgite composites were shown to be a better adsorbent for Fe(III) (KF: 7.40 to 13.77 mg g−1 at 298 to 318 K) than Cr(III) (KF: 4.31 to 13.62 mg g−1 at 298 to 318 K).132 The synergistic effect of broadened pores and electrostatic interactions between composites and the metal ions was suggested to have an important role.
The silico-antimonate ion exchanger had KF of 0.79, 0.44, 0.93 and 0.47 0.44 mmol g−1 respectively for Cd(II), Cu(II), Ni(II) and Zn(II) from water (adsorbent 10.0 g L−1, metal ions 5 × 10−2 to 10−4 M, pH 2.0, 298 K).133 The interactions were essentially those between a Lewis base and a Lewis acid. The interactions of the metal ions with the hydrated Si(IV) antimonate, increased with an increase in the ionic radii and the order of selectivity for the metal ions was Ni(II) > Cd(II) > Zn(II) > Cu(II). When Fe(III)–titanate ion exchanger was used for Cd(II) and Zn(II) adsorption,134 Freundlich capacities were 3.98 × 10−3 and 7.97 × 10−3 mol1−1/n L1/n g−1 respectively at 298 K. The small radius of dehydrated Zn(II) increased the mobility and the packing density of Zn(II) compared to Cd(II), facilitating the greater uptake of Zn(II).
Co(II) adsorption on two ion exchange resins (IRN77 and SKN1) had Freundlich capacities of 75.63 and 60.03 mg g−1.135
Shaaban et al.136 reported that Hg(II) (1.158 mmol L−1) was more preferably removed by dithiocarbamate chelating resin in comparison to Cd(II) (0.831 mmol L−1) and Pb(II) (0.588 mmol L−1). Through the study of surface morphology, it was observed that chelating resin before adsorption had least packed structure with many pores or cavities in the surface, which facilitated mass transfer of metal ions to the adsorbent.
Increase in KF from 0.44 to1.09 and 0.57 to 1.82 L1/n g−1 mg1−1/n for Cu(II) and Pb(II) on electric furnace slag was observed in the temperature interval of 293 to 313 K.137 The mixture of calcium-ferrite phases [CaO·Fe2O3, CaO·2Fe2O3, CaO·7Fe2O3] and calcium-manganese-silicates phase [(Ca, Mn)2·SiO4, CaO·MgO·SiO2] on the surface of the slag was suggested as being responsible for the cation uptake.
Activated red mud had a small KF for As(III) of 0.37 L μmol−1 while the values for As(V) were 5.93 to 63.27 L μmol−1.138 The adsorbent was shown to have highly porous aggregates of much finer particles with large number of adsorption sites.
Gupta et al.139 used a carbon slurry (fertilizer industry waste) for Cr(VI) uptake and reported KF values in the range of 1.09 to 1.02 mg g−1. The adsorbent contained constituents such as C (78.93%), O (19.41%), Al (1.16%) and F (0.49%). The organic content generally imparted porosity to the adsorbent. The large surface of the slurry enhanced the metal uptake capacity.
Freundlich capacity of Cd(II) on waste material from boron enrichment process was higher than Zn(II) removal (Cd(II): 1.75, Zn(II) 1.44 mg g−1).140 The authors reported that buexite (NaCaB5O9·8H2O) was the major phase of the adsorbent, where Ca was reduced significantly after adsorption of Cd(II) and Zn(II), suggesting ion exchange occurring between Ca2+ and Cd(II)/Zn(II).
2.2. Langmuir isotherm and its applications
Langmuir model141 was originally developed to describe gas adsorption onto activated carbon. It is the most straightforward non-linear isotherm based on the assumptions that (i) the adsorbent surface has energetically uniform sites for adsorbate ions, atoms and molecules, (ii) no adsorbate–adsorbate interactions are present, (iii) the same mechanism is followed throughout the process, and (iii) at the maximum adsorption, the surface is covered with only a monolayer.Langmuir model was derived by following mass action, kinetic, or statistical thermodynamic approaches. The isotherm refers to homogeneous adsorption, where each interacting molecule carries constant enthalpy and activation energy.142 Moreover, it is assumed that no transmigration of the adsorbate in the plane of the surface occurs during the interaction process.3,143
Langmuir isotherm equation is based on evaluating the fractional surface coverage, θ
| θ = qe/qm = bCe/(1 + bCe) | (3) |
| Ce/qe = (1/bqm) + (1/qm)Ce | (4) |
Plots of Ce/qe vs. Ce are utilized to obtain the values of qm and b.
Langmuir capacities provide a useful and convenient method to compare the adsorption capacities of similar or different types of solids. Because of the ease of application, almost every work on adsorption reports on Langmuir isotherm and the two coefficients, qm and b. The Langmuir capacities for adsorption of metal ions on some inorganic adsorbents are given in Table 3. A selection of recent literature on application of Langmuir isotherm is discussed below.
| Metal cation/adsorbent | Experimental variables | Langmuir capacity (qm) | Ref. | ||
|---|---|---|---|---|---|
| As(III) | |||||
| Octadecyl benzyl dimethyl ammonium modified bentonite | 306 K | 0.82 | mg g−1 | 68 | |
| Natural muscovite | 293 K | As(III) 0–100 mg L−1, pH 6.0 | 0.330 | mg g−1 | 43 |
| Hematite | pH 7.3 | (9.3 ± 0.2) × 10−6 | mol m−2 | 211 | |
| Magnetite | pH 6.5 | (3.1 ± 0.1) × 10−6 | |||
| Goethite | pH 7.5 | (2.5 ± 0.1) × 10−6 | |||
| Fe modified beidellite | 298 K | As(III) 250–5000 μg L−1 | 789.9 | μg g−1 | 181 |
| 308 K | 808.4 | ||||
| 318 K | 834.0 | ||||
| Fe–Ce modified zeolite | 298 K | 476.6 | |||
| 308 K | 499.3 | ||||
| 318 K | 540.5 | ||||
| Fe modified sepiolite | 298 K | 477.3 | |||
| 308 K | 490.7 | ||||
| 318 K | 512.3 | ||||
| Fe(NO3)3 modified zeolite | As(III) 0–70 μM | 40.48 | μmol L−1 | 187 | |
| Polymetallic sea nodule | As(III) 0.2 mg L−1 | 0.69 | mg g−1 | 222 | |
| As(III) 0.15 mg L−1 | 0.58 | ||||
| As(III) 0.1 mg L−1 | 0.31 | ||||
| MnFe2O4 | 293 K | pH 7.0 | 93.8 | mg g−1 | 104 |
| CoFe2O4 | 100.3 | ||||
| Fe3O4 | 49.8 | ||||
| Sand | 293 K | As(III) 100–800 μg L−1, pH 7.5 | 5.63 | μg g−1 | 216 |
| Iron oxide-coated sand | 28.57 | ||||
| Activated red mud | 296 ± 1 K | As(III) 2.04–156.66 μM, pH 6.8 ± 0.1 | 7.22 | mmol g−1 | 138 |
| As(V) | |||||
| Octadecyl benzyl dimethyl ammonium modified bentonite | 306 K | 1.48 | mg g−1 | 68 | |
| Natural muscovite | 293 K | As(V) 0–100 mg L−1, pH 6.0 | 0.791 | mg g−1 | 43 |
| Laterite | 305 K | As(V) 0.5–4 mg L−1, pH 5.5 | 0.149 | mg g−1 | 208 |
| As(V) 4.0–20 mg L−1, pH 5.5 | 0.306 | ||||
| As(V) 0.2–4 mg L−1, pH 7.0 | 0.140 | ||||
| As(V) 4.0–20 mg L−1, pH 7.0 | 0.240 | ||||
| As(V) 0.2–20 mg L−1, pH 5.5 | 0.507 | ||||
| 293 K | As(V) 0.5–4 mg L−1, pH 7.0 | 0.130 | |||
| As(V) 4.0–20 mg L−1, pH 7.0 | 0.220 | ||||
| 315 K | As(V) 0.5–5 mg L−1, pH 5.5 | 0.238 | |||
| As(V) 5.0–20 mg L−1, pH 5.5 | 0.569 | ||||
| As(V) 0.5–4 mg L−1, pH 7.0 | 0.156 | ||||
| As(V) 4.0–20 mg L−1, pH 7.0 | 0.275 | ||||
| As(V) 0.5–20 mg L−1, pH 5.5 | 0.565 | ||||
| Hematite | pH 7.3 | (2.9 ± 0.5) × 10−5 | mol m−2 | 211 | |
| Magnetite | pH 6.5 | (3.8 ± 0.2) × 10−6 | |||
| Goethite | pH 7.5 | (3.0 ± 0.2) × 10−6 | |||
| Iron oxide-coated perlite | 303 K | pH 6.5–7.0 | 0.39 | mg g−1 | 161 |
| Fe modified beydellite | 298 K | As(III) 250–5000 μg L−1 | 794.3 | μg g−1 | 181 |
| 308 K | 817.6 | ||||
| 318 K | 841.0 | ||||
| Fe–Ce modified zeolite | 298 K | 542.3 | |||
| 308 K | 547.3 | ||||
| 318 K | 533.4 | ||||
| Fe modified sepiolite | 298 K | 515.7 | |||
| 308 K | 538.8 | ||||
| 318 K | 558.0 | ||||
| Fe3O4 | 0.35 | mmol g−1 | 201 | ||
| CeO2 | 0.45 | ||||
| Fe–Ce bimetal oxide | 2.00 | ||||
| MnFe2O4 | 293 K | pH 3.0 | 90.4 | mg g−1 | 104 |
| CoFe2O4 | 73.8 | ||||
| Fe3O4 | 44.1 | ||||
| Ce(IV)-doped iron oxide | 293 K | 70.4 | mg g−1 | 105 | |
| Cetyltrimethylammonium bromide modified Fe3O4 | 293 K | As(V) 100 μg L−1 to 7 mg L−1 | 13.21 | mg g−1 | 108 |
| Phosphonic acid modified silica polyamine composite | pH 4.0 | 98.00 | mg g−1 | 191 | |
| pH 6.0 | 55.00 | ||||
| Chitosan–clay–magnetite composite | 296 ± 2 K | pH 5.0 | 6.5 | mg g−1 | 178 |
| LDH intercalated by Cl− | As(VI) 0.02–1.0 mmol L−1 | 322.58 | mmol kg−1 | 203 | |
| Gibbsite | 51.55 | ||||
| Polymetallic sea nodule | As(V) 0.24 mg L−1 | 2.85 | mg g−1 | 222 | |
| As(V) 0.16 mg L−1 | 1.47 | ||||
| As(V) 0.1 mg L−1 | 10.2 | ||||
| Activated red mud | 288 K | As(V) 5.74–182.57 μM, pH 7.0 ± 0.1 | 25.91 | mmol g−1 | 138 |
| 293 K | As(V) 7.70–136.50 μM, pH 7.0 ± 0.1 | 28.90 | |||
| 296 ± 1 K | As(V) 2.0–151.87 μM, pH 7.0 ± 0.1 | 39.84 | |||
| 323 K | As(V) 6.91–152.18 μM, pH 7.0 ± 0.1 | 40.98 | |||
| 296 ± 1 K | As(V) 7.03–220.85 μM, pH 4.5 ± 0.1 | 102.00 | |||
| Cd(II) | |||||
| Kaolin | 303 K | 6.71 × 10−3 | mmol g−1 | 27 | |
| Ball clay | 2.10 × 10−2 | ||||
| Bentonite | 303 K | Cd(II) 50–300 mg L−1 | 6.80 × 102 | mmol g−1 | 36 |
| Montmorillonite K 10 | 298 K | 6.30 | mg g−1 | 33 | |
| Brazilian bentonite | 11.20 | ||||
| Acid-activated kaolinite | 303 K | pH 5.5 | 11.70 | mg g−1 | 46 |
| Acid-activated montmorillonite | 33.20 | ||||
| Sepiolite | 293 ± 1 K | 0.388 | meq g−1 | 151 | |
| Fe-montmorillonite | 298 K | pH 5.0 | 25.70 | mg g−1 | 65 |
| Phosphate-immobilized Zr-pillared bentonite | pH 6.0 | 50.07 | mg g−1 | 159 | |
| Montmorillonite | 293 K | 21.90 | μM kg−1 | 26 | |
| Hydroxy-Al interlayered montmorillonite | 2.50 | ||||
| Na+-montmorillonite | pH 6.5 | 0.125 | mmol kg−1 | 64 | |
| Acid-activated Na+-montmorillonite | 0.139 | ||||
| Al13-pillared acid-activated Na+-montmorillonite | 0.163 | ||||
| Bentonite | 293 K | pH 5.0 | 61.35 | mg g−1 | 61 |
Bentonite/iron oxides (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 20 °C) 1 at 20 °C) |
63.29 | ||||
Bentonite/iron oxides (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 85 °C) 1 at 85 °C) |
48.78 | ||||
| Fe-pillared bentonite | Cd(II) 0.178–5.34 mmol L−1 | 0.773 | mmol g−1 | 63 | |
| Fe/Cr-pillared bentonite | 0.633 | ||||
| Cr-pillared bentonite | 0.423 | ||||
| Montmorillonite | 298 K | pH 5.0 | 11.868 | mg g−1 | 66 |
| Humic acid modified Ca-montmorillonite | 14.148 | ||||
| Montmorillonite | 295 K | pH 5.0 | 8.06 | mg g−1 | 163 |
| Sodium dodecyl sulphate-montmorillonite | 11.67 | ||||
| Hydroxy-alumino-silicate-montmorillonite | 14.36 | ||||
| Kaolinite | 303 K | pH 5.5 | 6.78 | mg g−1 | 47 |
| ZrO-kaolinite | 5.27 | ||||
| TBA-kaolinite | 6.31 | ||||
| Montmorillonite | 30.67 | ||||
| ZrO-montmorillonite | 36.63 | ||||
| TBA-montmorillonite | 43.47 | ||||
| Bentonite | 303 K | pH 6.02 | 7.73 | mg g−1 | 58 |
| 323 K | 11.26 | ||||
| Bentonite + goethite | 303 K | 9.56 | |||
| 323 K | 12.34 | ||||
| Bentonite + humic acid | 303 K | 9.90 | |||
| 323 K | 15.92 | ||||
| Bentonite + geothite + humic acid | 303 K | 10.09 | |||
| 323 K | 16.00 | ||||
| Vermiculite | 298 K | Cd(II) 0–500 μM | 143.00 | μmol g−1 | 90 |
| Zeolite | 118.00 | ||||
| Pumice | 47.00 | ||||
| Natural muscovite | 293 K | Cd(II) 0–100 mg L−1, pH 6.0 | 0.750 | mg g−1 | 43 |
| Phosphate | 298 ± 1 K | 7.54 | mg g−1 | 209 | |
| Ca-deficient hydroxyapatite | Cd(II) 10–30 mg L−1 | 23.04 | mg g−1 | 177 | |
| N-2-Hydroxypropyl trimethyl ammonium chloride chitosan modified bentonite | 293 K | 22.23 | mg g−1 | 169 | |
| Zeolite-based geopolymer | — | — | 26.246 | mg g−1 | 89 |
| Tannin immobilised calcined hydrotalcite | 303 K | pH 6.0 | 78.9 | mg g−1 | 118 |
| 313 K | 81.9 | ||||
| 323 K | 91.5 | ||||
| 333 K | 101.7 | ||||
| Mixed oxide [0.5 M SiO2–0.5 M Fe(OH)3] | 303 K | pH 5.0 | 9.81 × 10−2 | mmol g−1 | 199 |
| 313 K | 10.23 × 10−2 | ||||
| 323 K | 10.64 × 10−2 | ||||
| SiO2 | 288 K | Cd(II) 8.89 × 10−2 to 88.9 × 10−2 mmol L−1 | 0.033 | mmol g−1 | 198 |
| 298 K | 0.036 | ||||
| 308 K | 0.039 | ||||
| 318 K | 0.043 | ||||
| Fe(OH)3 | 288 K | 0.086 | |||
| 298 K | 0.089 | ||||
| 308 K | 0.091 | ||||
| 318 K | 0.094 | ||||
| Mixed oxide [0.5 M SiO2–0.5 M Fe(OH)3] | 288 K | 0.101 | |||
| 298 K | 0.103 | ||||
| 308 K | 0.107 | ||||
| 318 K | 0.112 | ||||
| Thiol-functionalized silica | 298 K | Cd(II) 20–250 mg L−1 | 33.72 ± 1.23 | mg g−1 | 117 |
| Amino functionalized MCM-41 | Cd(II) 10–70 mg L−1 | 18.25 | mg g−1 | 116 | |
| Poly(ethyleneimine)–silica gel | pH 5.0–6.0 | 38.46 | mg g−1 | 192 | |
| Glutaraldehyde crosslinked poly(ethyleneimine)–silica gel | 29.26 | ||||
| Nano zerovalent iron | 285 K | Cd(II) 25–450 mg L−1 | 714.3 | mg g−1 | 218 |
| 297 K | 769.2 | ||||
| 307 K | 714.3 | ||||
| Boehmite (γ-AlOOH) | 298 K | Cd(II) 50 mg L−1, pH 6.0 | 3.552 | mg g−1 | 114 |
| Goethite (γ-FeOOH) | 5.276 | ||||
| Iron oxide-immobilized sand | 298 ± 1 K | 0.528 | mg g−1 | 128 | |
| Loess soils | 278 K | Cd(II) 25–150 mg L−1 | 6.68 | mg g−1 | 123 |
| 288 K | 7.03 | ||||
| 298 K | 8.17 | ||||
| 308 K | 8.90 | ||||
| 318 K | 9.37 | ||||
| Hydrous MnO2 | 298 K | pH 3.5–4.0 | 12.49 | mmol g−1 | 96 |
| NiO | 303 K | Cd(II) 100–600 mg L−1 | 625.0 | mg g−1 | 94 |
| Al2O3 | 303 K | pH 6.7 | 89.28 | mg g−1 | 95 |
| Activated alumina | 303 K | pH 5.0 | 35.06 | mg g−1 | 111 |
| Titanate nanotube | 298 ± 1 K | Cd(II) 0.1–3.0 mmol L−1 | 2.13 | mmol g−1 | 99 |
| Fly ash | 303 K | 1.24 | mg g−1 | 231 | |
| 313 K | 1.64 | ||||
| 323 K | 2.00 | ||||
| Vanadium mine tailing | 303 K | 3.52 | mg g−1 | 223 | |
| 313 K | 4.49 | ||||
| 323 K | 8.83 | ||||
| Clarified sludge | 303 ± 0.5 K | pH 5.0 | 36.23 | mg g−1 | 240 |
| Dithiocarbamate chelating resin | 298 K | Cd(II) 1–25 mmol L−1 | 2.15 | mmol g−1 | 136 |
| Co(II) | |||||
| Kaolinite | 303 K | pH 5.8 | 11.2 | mg g−1 | 50 |
| Acid-activated kaolinite | 12.1 | ||||
| Montmorillonite | 28.6 | ||||
| Acid-activated montmorillonite | 29.7 | ||||
| Bentonite | 293 K | pH 3.0 | 6.775 | mg g−1 | 34 |
| pH 5.0 | 8.467 | ||||
| pH 7.0 | 9.911 | ||||
| pH 9.0 | 16.863 | ||||
| HCl/NaOH-treated bentonite | 295 ± 1 K | Co(II) 20–200 mg L−1 | 138.17 | mg g−1 | 45 |
| Phosphate-immobilized Zr-pillared bentonite | pH 6.0 | 47.81 | mg g−1 | 159 | |
| Humic acid-immobilized-amine modified polyacrylamide–bentonite composite | 303 K | pH 8.0 | 106.21 | mg g−1 | 168 |
| Smectite | 298 ± 1 K | pH 5.0 | (13.9 ± 0.1) × 103 | mmol g−1 | 167 |
| Aluminum pillared/3-mercap-topropyltrimethoxysilane organofunctionalized smectite | (23.8 ± 0.1) × 103 | ||||
| Zirconium pillared/3-mercap-topropyltrimethoxysilane organofunctionalized smectites | (25.8 ± 0.2) × 103 | ||||
| ZrO-kaolinite | 303 K | Co(II) 10–250 mg L−1, pH 5.7 | 9.0 | mg g−1 | 49 |
| ZrO-montmorillonite | 22.3 | ||||
| TBA-kaolinite | 303 K | Co(II) 10–250 mg L−1, pH 5.7 | 8.4 | mg g−1 | 164 |
| TBA-montmorillonite | 19.7 | ||||
| NiO | 303 K | pH 7.5 | 8.13 × 10−5 | mol g−1 | 195 |
| 308 K | 8.48 × 10−5 | ||||
| 313 K | 8.69 × 10−5 | ||||
| 323 K | 8.93 × 10−5 | ||||
| 303 ± 1 K | pH 7.0 | 6.41 × 10−5 | |||
| pH 7.5 | 8.13 × 10−5 | ||||
| pH 8.0 | 11.27 × 10−5 | ||||
| pH 8.5 | 17.61 × 10−5 | ||||
| Mg(OH)2 | 298 K | Co(II) 3–135 mg L−1 | 95.24 | mg g−1 | 196 |
| 308 K | 117.65 | ||||
| 318 K | 125.00 | ||||
| Clinoptilolite | 303 K | Co(II) 100–400 mg L−1 | 244.13 | mg g−1 | 182 |
| Cancrinite-type zeolite | 298 ± 2.1 K | 1.532 | mmol g−1 | 185 | |
| Ion exchange resins IRN77 | 298 ± 1 K | 86.17 | mg g−1 | 135 | |
| SKN1 | 69.44 | ||||
| Cr(III) | |||||
| Kaolinite | 303 K | pH 5.5, ionic strength 1 × 10−3 | 4.40 × 10−5 | mol g−1 | 28 |
| 4.47 × 10−5 | |||||
| 4.98 × 10−5 | |||||
| 313 K | pH 5.5, ionic strength 1 × 10−3 | 4.40 × 10−5 | |||
| 323 K | |||||
| 303 K | |||||
| pH 5.5, ionic strength 1 × 10−2 | 4.51 × 10−5 | ||||
| pH 5.5, ionic strength 1 × 10−1 | 5.83 × 10−5 | ||||
| 303 K | pH 4.5 | 3.86 × 10−5 | |||
| pH 5.0 | 4.09 × 10−5 | ||||
| pH 5.5 | 6.62 × 10−5 | ||||
| pH 6.0 | 4.34 × 10−5 | ||||
| Turkish kaolinite | 293 K | pH 6.0 | 21.55 | mg g−1 | 31 |
| Kaolin | 303 K | 3.49 × 10−2 | mmol g−1 | 27 | |
| Ball clay | 6.90 × 10−2 | ||||
| Montmorillonite | 295 K | pH 5.0 | 11.00 | mg g−1 | 163 |
| Sodium dodecyl sulphate-montmorillonite | 13.28 | ||||
| Hydroxy-alumino-silicate-montmorillonite | 14.83 | ||||
| Montmorillonite | 298 K | pH 5.0 | 12.443 | mg g−1 | 66 |
| Humic acid modified Ca-montmorillonite | 15.657 | ||||
| Al-/cetyl trimethyl ammonium-modified montmorillonite | 2.28 × 104 | mol g−1 | 67 | ||
| Ca-montmorillonite | 298 K | pH 5.0 | 12.443 | mg g−1 | 65 |
| Humic acid modified Ca-montmorillonite | 15.657 | ||||
| Vermiculite | 298 K | Adsorbent 1.25 g L−1 | 46.95 | mg g−1 | 153 |
| Sepiolite | 293 ± 1 K | Adsorbent 2.5–25 g L−1 | 0.534 | meq g−1 | 151 |
| Chitosan–attapulgite composites | 298 K | 27.03 | mg g−1 | 132 | |
| 308 K | 46.08 | ||||
| 318 K | 65.36 | ||||
| Hydrous TiO2 | 288 K | pH 5.0 | 14.03 | mg g−1 | 255 |
| 303 K | 17.73 | ||||
| 318 K | 17.45 | ||||
| Titanate nanotube | 298 ± 1 K | Cr(III) 0.1–3.0 mmol L−1 | 1.37 | mmol g−1 | 80 |
| Vanadium mine tailing | 303 K | 16.23 | mg g−1 | 223 | |
| 313 K | 19.33 | ||||
| 323 K | 28.27 | ||||
| Bagasse fly ash | 303 K | 4.35 | mg g−1 | 229 | |
| 313 K | 4.30 | ||||
| 323 K | 4.25 | ||||
| Cr(VI) | |||||
| Turkish vermiculite | 293 K | pH 1.5 | 87.7 | mg g−1 | 250 |
| Kaolinite | 303 K | Cr(VI), 10–250 mg L−1, pH 4.6 | 11.60 | mg g−1 | 55 |
| Acid-activated kaolinite | 13.90 | ||||
| ZrO-kaolinite | 10.90 | ||||
| TBA-kaolinite | 10.60 | ||||
Alkyl ammonium surfactant bentonite (2.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clay–surfactant (w/w) ratio 1 clay–surfactant (w/w) ratio |
291 K | 8.36 | mg g−1 | 165 | |
| 13.57 | |||||
Alkyl ammonium surfactant bentonite (4.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clay–surfactant (w/w) ratio) 1 clay–surfactant (w/w) ratio) |
310 K | 8.51 | |||
| 14.64 | |||||
| Spent activated clay | 277 K | pH 2.0 | 0.743 | mg g−1 | 149 |
| pH 2.5 | 0.589 | ||||
| pH 3.0 | 0.502 | ||||
| pH 3.5 | 0.427 | ||||
| pH 4.0 | 0.331 | ||||
| 287 K | pH 2.0 | 0.756 | |||
| pH 2.5 | 0.618 | ||||
| pH 3.0 | 0.518 | ||||
| pH 3.5 | 0.436 | ||||
| pH 4.0 | 0.327 | ||||
| 297 K | pH 2.0 | 0.957 | |||
| pH 2.5 | 0.621 | ||||
| pH 3.0 | 0.526 | ||||
| pH 3.5 | 0.482 | ||||
| pH 4.0 | 0.331 | ||||
| 313 K | pH 2.0 | 1.422 | |||
| pH 2.5 | 0.649 | ||||
| pH 3.0 | 0.557 | ||||
| pH 3.5 | 0.548 | ||||
| pH 4.0 | 0.334 | ||||
| Tannin-immobilized activated clay | 300 K | 18.34 | mg g−1 | 76 | |
| 310 K | 20.12 | ||||
| 320 K | 21.19 | ||||
| 330 K | 24.09 | ||||
| [3-(2-Aminoethylamino)propyl trimethoxy-silane] grafted natural sepiolite | 298 K | Cr(VI) 10–250 mg L−1 | 52.99 | mg g−1 | 78 |
| [3-(2-Aminoethylamino)propyl trimethoxy-silane] grafted acid-activated sepiolite | 80.84 | ||||
| Activated alumina | pH 3.0 | 25.57 | mg g−1 | 239 | |
| Fuller's earth | 23.58 | ||||
| Dolomite | 293 K | pH 2.0 | 10.010 | mg g−1 | 205 |
| 303 K | 8.385 | ||||
| 313 K | 6.654 | ||||
| 333 K | 5.609 | ||||
| Hexa decyl trimethyl ammonium-zeolite | 288 K | pH 6.0 | 5.07 | mg g−1 | 188 |
| 281 K | pH 8.0 | 3.10 | |||
| 283 K | pH 10.0 | 0.21 | |||
| Dodecyl benzyl dimethyl ammonium-rectorite | 299 K | pH 6.0 | 0.97 | mg g−1 | 215 |
| Hexadecyl trimethyl ammonium-rectorite | 2.39 | ||||
| Octadecyl trimethyl ammonium-rectorite | 3.57 | ||||
| Hydrous TiO2 | 288 K | pH 1.5 | 10.83 | mg g−1 | 255 |
| 303 K | 20.00 | ||||
| 318 K | 26.81 | ||||
| Hydrous ZrO2 | 298 | pH 2.0 | 61.00 | mg g−1 | 91 |
| 308 | 61.00 | ||||
| 318 | 63.00 | ||||
| 328 | 64.00 | ||||
| 338 | 66.00 | ||||
| Montmorillonite-supported magnetite | 298 ± 2 K | 13.88 | mg g−1 | 219 | |
| Magnetite | 298 ± 2 K | 20.16 | mg g−1 | 220 | |
| Commercial magnetite | 13.72 | ||||
| Diatomite-supported magnetite | 12.31 | ||||
| Loamy sand soil | 1570.00 | mg kg−1 | 124 | ||
| Zero-valent iron | 298 K | Cr(VI) 60–100 mg L−1 | 76.92 | mg g−1 | 62 |
| Fe–Ni bimetallic nanoparticle | 100.00 | ||||
| Fe–Ni bimetallic-montmorillonite nanocomposite | 100.00 | ||||
| Red mud | 300 K | pH 2.0 | 4.36 × 10−4 | mol g−1 | 237 |
| 313 K | 4.15 × 10−4 | ||||
| 323 K | 4.05 × 10−4 | ||||
| Fly ash | pH 3.0 | 23.86 | mg g−1 | 239 | |
| Clarified sludge | 26.31 | ||||
| Fly ash | 1.379 | mg g−1 | 235 | ||
| Fly ash impregnated with Al | 1.820 | ||||
| Fly ash impregnated with Fe | 1.667 | ||||
| Cu(II) | |||||
| Kaolinite | 293 K | 14.89 | mg g−1 | 249 | |
| 303 K | 16.75 | ||||
| 313 K | 16.79 | ||||
| Kaolin | 303 K | 12.00 × 10−3 | mmol g−1 | 27 | |
| Ball clay | 2.50 × 10−2 | ||||
| Local bentonite | 293 K | 909 | mg g−1 | 145 | |
| Local bentonite | 293.15 K | 9.73 × 10−5 | mol g−1 | 37 | |
| 313.15 K | 9.65 × 10−5 | ||||
| 333.15 K | 1.08 × 10−5 | ||||
| Local montmorillonite | 30.994 | mg g−1 | 252 | ||
| Cankiri bentonite | 296 K | 44.84 | mg g−1 | 39 | |
| Bentonite | 293 K | pH 3.0 | 12.376 | mg g−1 | 34 |
| pH 5.0 | 14.104 | ||||
| pH 7.0 | 12.547 | ||||
| pH 9.0 | 15.924 | ||||
| Bentonite | 303 K | Cu(II) 50–300 mg L−1 | 12.5 × 102 | mmol g−1 | 36 |
| Na-bentonite | 298 K | 17.877 | mg g−1 | 147 | |
| Natural kaolinite | 298 ± 1 K | 6.2 ± 0.2 | mmol g−1 | 154 | |
| Urea-intercalated kaolinite | 8.5 ± 0.1 | ||||
| Urea intercalated/delaminated kaolinite | 9.6 ± 0.2 | ||||
| Kaolinite | 303 K | Cu(II) 10–250 mg L−1, pH 5.7 | 9.2 | mg g−1 | 57 |
| Acid-activated kaolinite | 10.1 | ||||
| Montmorillonite | 31.8 | ||||
| Acid-activated montmorillonite | 32.3 | ||||
| Montmorillonite | 298 K | pH 5.0 | 12.633 | mg g−1 | 66 |
| Humic acid modified Ca-montmorillonite | 15.254 | ||||
| Bentonite | Cu(II) 200–700 mg L−1 | 149.25 | mg g−1 | 160 | |
| Phosphate-modified bentonite | 158.73 | ||||
| Sulphate-modified bentonite | 166.67 | ||||
| MgO coated bentonite | 295.15 K | pH 6.0 | 58.44 ± 0.46 | mg g−1 | 59 |
| Ca-montmorillonite | 298 K | pH 5.0 | 12.443 | mg g−1 | 66 |
| Humic acid modified Ca-montmorillonite | 15.657 | ||||
| Montmorillonite | 295 K | pH 5.0 | 10.09 | mg g−1 | 163 |
| Sodium dodecyl sulphate-montmorillonite | 12.27 | ||||
| Hydroxy-alumino-silicate-montmorillonite | 14.51 | ||||
| Sodium dodecyl sulfate montmorillonite | Cu(II) 4.5–45 mmol | 254.1 | mmol kg−1 | 171 | |
| Smectite | 298 ± 1 K | pH 5.0 | (15.7 ± 0.1) × 103 | mmol g−1 | 167 |
| Aluminum pillared/3-mercapto propyltrimethoxysilane organofunctionalized smectite | (28.2 ± 0.1) × 103 | ||||
| Zirconium pillared and 3-mercapto propyltrimethoxy-silane organofunctionalized smectite | (29.4 ± 0.1) × 103 | ||||
| ZrO-kaolinite | 303 K | Cu(II) 10–250 mg L−1, pH 5.7 | 3.0 | mg g−1 | 56 |
| TBA-kaolinite | 3.2 | ||||
| ZrO-montmorillonite | 7.1 | ||||
| TBA-montmorillonite | 27.3 | ||||
| Bentonite | 303 K | pH 6.02 | 9.72 | mg g−1 | 58 |
| 323 K | 11.29 | ||||
| Bentonite + goethite | 303 K | 9.90 | |||
| 323 K | 13.91 | ||||
| Bentonite + humic acid | 303 K | 10.33 | |||
| 323 K | 16.13 | ||||
| Bentonite + geothite + humic acid | 303 K | 10.03 | |||
| 323 K | |||||
| 16.42 | |||||
| 8-Hydroxy Quinoline immobilized bentonite | 293 K | 51.12 | mg g−1 | 70 | |
| 303 K | 51.56 | ||||
| 313 K | 55.04 | ||||
| 323 K | 56.55 | ||||
| 2,2′-Dipyridyl immobilized bentonite | 293 K | Cu(II) 92.5–200 mg L−1, pH 5.7 | 49.44 | mg g−1 | 72 |
| 303 K | 50.33 | ||||
| 313 K | 54.01 | ||||
| 323 K | 54.07 | ||||
| Chitosan immobilized bentonite | Cu(II) 50–500 mg L−1, pH 4.0 | 21.552 | mg g−1 | 176 | |
| Humic acid-immobilized-amine modified polyacrylamide–bentonite composite | 303 K | pH 8.0 | 96.15 | mg g−1 | 168 |
| Bentonite | 293 ± 2 K | pH 5.0 | 11.36 | mg g−1 | 177 |
| pH 6.2 | 29.43 | ||||
| Bentonite–polyacrylamide composite | pH 5.0 | 19.89 | |||
| pH 6.2 | 32.81 | ||||
| Chitosan–clay–magnetite composite | 296 ± 2 K | pH 5.0 | 14.30 | mg g−1 | 178 |
| Vermiculite | 298 K | Adsorbent 1.25 g L−1 | 43.67 | mg g−1 | 153 |
| Natural muscovite | 293 K | Cu(II) 0–100 mg L−1, pH 6.0 | 0.618 | mg g−1 | 43 |
| Local zeolite | 0.128 | mmol g−1 | 180 | ||
| Local clay | 0.098 | ||||
| Local diatomite | 0.047 | ||||
| Spent activated clay | 300 K | pH 5.0 ± 0.1 | 10.9 | mg g−1 | 148 |
| pH 5.5 ± 0.1 | 11.5 | ||||
| pH 6.0 ± 0.1 | 13.2 | ||||
| Tannin immobilized calcined hydrotalcite | 303 K | pH 6.0 | 81.4 | mg g−1 | 118 |
| 313 K | 84.4 | ||||
| 323 K | 94.7 | ||||
| 333 K | 103.5 | ||||
| Iron oxide-immobilized sand | 298 ± 1 K | 1.265 | mg g−1 | 128 | |
| Natural zeolite tuff | 303 K | pH 5.0 | 23.3 | mg g−1 | 83 |
| Clinoptilolite | 303 K | Cu(II) 100–400 mg L−1 | 141.12 | mg g−1 | 182 |
| Humic acid-immobilized cetylpyridinium-zeolite | 298 K | 19.8 | mg g−1 | 88 | |
| 303 K | 20.5 | ||||
| 318 K | 21.5 | ||||
| Cancrinite-type zeolite | 298 ± 2.1 K | 2.081 | mmol g−1 | 185 | |
| N-[3-(Trimethoxysilyl)propyl]-ethylenediamine grafted silica | 293 K | 0.182 | mmol g−1 | 193 | |
Amino functionalized silica gel (3-aminopropyltri-methoxysilane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica = 0.1) silica = 0.1) |
0.485 | mmol g−1 | 115 | ||
Amino functionalized silica gel (3-aminopropyltri-methoxysilane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica = 0.2) silica = 0.2) |
0.500 | ||||
Amino functionalized silica gel (3-aminopropyltri-methoxysilane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica = 0.3) silica = 0.3) |
0.850 | ||||
| Aminated mesoporous alumina | 298 ± 1 K | 7.09 | mg g−1 | 112 | |
| Protonated mesoporous alumina | 8.55 | ||||
| 3-Aminopropyltriethoxysilane/glutaraldehyde coated Fe3O4 | 293 ± 1 K | pH 4.0 | 61.07 | mg g−1 | 107 |
| Titanate nanotube | 298 ± 1 K | Cu(II) 0.1–3.0 mmol L−1 | 1.92 | mmol g−1 | 99 |
| Powered limestone | 298 K | 0.29 | mg g−1 | 226 | |
| Vanadium mine tailing | 303 K | 5.36 | mg g−1 | 223 | |
| 313 K | 8.75 | ||||
| 323 K | 14.96 | ||||
| Bagasse fly ash | 303 K | 2.26 | mg g−1 | 230 | |
| 313 K | 2.34 | ||||
| 323 K | 2.36 | ||||
| Fly ash | 303 K | 5.71 | mg g−1 | 232 | |
| 313 K | 5.67 | ||||
| 323 K | 5.56 | ||||
| Pit coal fly ash | 277 K | 4.594 | mg g−1 | 233 | |
| 291 K | 4.715 | ||||
| 333 K | 7.613 | ||||
| Electric furnace slag | 293 K | 32.68 | mg g−1 | 137 | |
| 303 K | 36.36 | ||||
| 313 K | 39.22 | ||||
| Fe(III) | |||||
| Kaolinite | 303 K | Fe(III) 10–250 mg L−1, pH 3.0 | 11.2 | mg g−1 | 52 |
| Acid-activated kaolinite | 12.1 | ||||
| Montmorillonite | 28.9 | ||||
| Acid-activated montmorillonite | 30.0 | ||||
| ZrO-kaolinite | 303 K | Fe(III) 10–250 mg L−1, pH 3.0 | 9.7 | mg g−1 | 49 |
| ZrO-montmorillonite | 23.8 | ||||
| TBA-kaolinite | 303 K | Fe(II) 10–250 mg L−1, pH 3.0 | 9.3 | mg g−1 | 164 |
| TBA-montmorillonite | 22.6 | ||||
| Chitosan–attapulgite composites | 298 K | 36.76 | mg g−1 | 132 | |
| 308 K | 47.17 | ||||
| 318 K | 62.50 | ||||
| Hg(II) | |||||
| Phosphate-immobilized Zr-pillared bentonite | pH 6.0 | 50.98 | mg g−1 | 159 | |
| Montmorillonite | 298.15 ± 0.2 K | pH 3.0 | 1.23 ± 0.12 | mmol g−1 | 173 |
| Dimethyl sulfoxide/3-aminopropyl-triethoxysilane intercalated montmorillonite | 1.56 ± 0.12 | ||||
| Pyridine/3-aminopropyl-triethoxysilane intercalated montmorillonite | 1.77 ± 0.12 | ||||
| Hybrid mesoporous aluminosilicate sieve | 303 ± 2 K | Hg(II) 2–16 mg L−1, pH 6.0 | 20.66 | mg g−1 | 80 |
| Silica gel-based hybrid composite (heterogeneous synthesis) | 288 K | Hg(II) 1 × 103 to 5 × 103 mol L−1 | 0.56 | mg g−1 | 194 |
| 298 K | 0.72 | ||||
| 308 K | 0.90 | ||||
| Silica gel-based hybrid composite (homogeneous synthesis) | 288 K | 0.57 | |||
| 298 K | 0.58 | ||||
| 308 K | 0.74 | ||||
| Dithiocarbamate chelating resin | 298 K | Hg(II) 1–25 mmol L−1 | 2.45 | mmol g−1 | 136 |
| Mn(II) | |||||
| Montmorillonite K 10 | 298 K | 4.80 | mg g−1 | 33 | |
| 6.00 | |||||
| Brazilian bentonite | |||||
| Sepiolite | 293 ± 1 K | Adsorbent 2.5–25 g L−1 | 0.247 | meq g−1 | 151 |
| Clinoptilolite | 303 K | Mn(II) 100–400 mg L−1 | 76.78 | mg g−1 | 182 |
| Natural nontronite | 298 ± 1 K | Mn(II) 0.125–2.5 mmol L−1 | 13.015 | mmol g−1 | 179 |
| 3-Aminopropyltriethoxy-silane modified nontronite | 23.256 | ||||
| Ni(II) | |||||
| Local bentonite | 293 K | 2.875 | mg g−1 | 150 | |
| 313 K | 3.072 | ||||
| 323 K | 3.047 | ||||
| 348 K | 3.893 | ||||
| Ball clay | 303 K | 7.00 × 10−3 | mmol g−1 | 27 | |
| Na-bentonite | 298 K | 13.966 | mg g−1 | 147 | |
| Chitosan immobilized bentonite | Ni(II) 50–500 mg L−1, pH 4.0 | 15.823 | mg g−1 | 176 | |
| Kaolinite | 303 K | Ni(II) 10–250 mg L−1, pH 5.7 | 10.4 | mg g−1 | 51 |
| Acid-activated kaolinite | 11.9 | ||||
| Montmorillonite | 28.4 | ||||
| Acid-activated montmorillonite | 29.5 | ||||
| ZrO-kaolinite | 303 K | Ni(II) 10–250 mg L−1, pH 5.7 | 8.8 | mg g−1 | 49 |
| ZrO-montmorillonite | 22.0 | ||||
| TBA-kaolinite | 303 K | Ni(II) 10–250 mg L−1, pH 5.7 | 8.4 | mg g−1 | 164 |
| TBA-montmorillonite | 19.7 | ||||
| Montmorillonite K10 | 298 K | 2.10 | mg g−1 | 74 | |
| 3-Mercaptopropyl-trimethoxysilane-modified montmorillonite K10 | 4.73 | ||||
| Na-attapulgite | 291.15 | pH 6.0 ± 0.1 | 9.42 × 10−5 | mol g−1 | 42 |
| 313.15 | 9.78 × 10−5 | ||||
| 333.15 | 1.05 × 10−5 | ||||
| Mg-mesoporous alumina | 298 ± 1 K | Ni(II) 5–30 mg L−1 | 22.32 | mg g−1 | 260 |
| Poly(ethyleneimine)–silica gel | pH 5.0–6.0 | 28.25 | mg g−1 | 192 | |
| Glutaraldehyde crosslinked poly(ethyleneimine)–silica gel | 18.62 | ||||
| Amino functionalized MCM-41 | Ni(II) 10–70 mg L−1 | 12.36 | mg g−1 | 116 | |
| Cancrinite-type zeolite | 298 ± 2.1 K | 1.532 | mmol g−1 | 185 | |
| Clinoptilolite | 293 K | 3.28 | mg g−1 | 85 | |
| 313 K | 2.97 | ||||
| 333 K | 2.65 | ||||
| Dimethylglyoxime treated clinoptilolite | 293 K | 0.96 | mmol g−1 | 87 | |
| 313 K | 0.18 | ||||
| 333 K | 0.13 | ||||
| Hydrous TiO2 | 288 ± 1 K | Ni(II) 10–150 mg L−1, pH 5.0 ± 1 | 19.18 | mg g−1 | 259 |
| 303 ± 1 K | 22.07 | ||||
| 318 ± 1 K | 23.24 | ||||
| 328 ± 1 K | 26.47 | ||||
| Fly ash | 303 K | 1.12 | mg g−1 | 231 | |
| 313 K | 1.35 | ||||
| 323 K | 1.70 | ||||
| Pb(II) | |||||
| Local bentonite | 293.13 | pH 5.2 ± 0.2 | 1.15 × 10−4 | mol g−1 | 32 |
| 323.13 | 1.46 × 10−4 | ||||
| 343.13 | 1.64 × 10−4 | ||||
| Turkish kaolinite | 293 K | pH 6.0 | 21.55 | mg g−1 | 31 |
| Turkish kaolinite | 293 K | pH 5.0 | 31.75 | mg g−1 | 30 |
| Kaolin | 303 K | 3.18 × 10−3 | mmol g−1 | 27 | |
| Ball clay | 1.70 × 10−2 | ||||
| Montmorillonite | 298 K | pH 6.0 | 57.0 | mg g−1 | 40 |
| Na-bentonite | 298 K | Pb(II) 1.45 × 10−5 to 1.74 × 10−4 mol L−1 | 2.31 × 10−4 | mol g−1 | 38 |
| 318 K | 2.52 × 10−4 | ||||
| 338 K | 3.10 × 10−4 | ||||
| Natural bentonite | 293 K | 107.00 | mg g−1 | 146 | |
| 313 K | 110.00 | ||||
| 333 K | 120.00 | ||||
| Acid-activated kaolinite | 303 K | Pb(II) 10–250 mg L−1, pH 5.7 | 12.33 | mg g−1 | 54 |
| Acid-activated montmorillonite | 31.35 | ||||
| Natural kaolinite | 298 ± 1 K | 6.3 ± 0.2 | mmol g−1 | 154 | |
| Urea-intercalated kaolinite | 9.6 ± 0.1 | ||||
| Urea intercalated and delaminated kaolinite | 9.9 ± 0.2 | ||||
| Kaolinite | 303 K | 4.73 | mg g−1 | 44 | |
| Aluminium sulphate modified kaolinite | 32.2 | ||||
| Kaolinite | 303 K | Pb(II) 10–250 mg L−1, pH 5.7 | 11.52 | mg g−1 | 53 |
| ZrO-kaolinite | 9.01 | ||||
| TBA-kaolinite | 5.44 | ||||
| Montmorillonite | 31.05 | ||||
| ZrO-montmorillonite | 31.44 | ||||
| TBA-montmorillonite | 30.67 | ||||
| Kaolinite | 295 ± 1 K | Pb(II) 50–400 mg L−1 | 13.32 | mg g−1 | 162 |
| Acid treated kaolinite | 51.60 | ||||
| NaOH/3-chloropropyl-triethoxysilane modified kaolinite | 54.35 | ||||
| Natural smectite | 298 ± 0.2 K | pH 55.0 mg L−1, pH 5.0 | 8.857 | mmol g−1 | 175 |
| Al-pillared 3-amino-propyltriethoxysilane organofunctionalized smectite | 9.831 | ||||
| Ti-pillared 3-amino-propytriethoxysilane organofunctionalized smectite | 9.546 | ||||
| Cr-pillared 3-amino-propyltriethoxysilane organofunctionalized smectite | 9.278 | ||||
| Zr-pillared 3-amino-propyltriethoxysilane organofunctionalized smectite | 9.347 | ||||
| Chitosan immobilized bentonite | Pb(II) 50–500 mg L−1, pH 4.0 | 26.385 | mg g−1 | 176 | |
| 8-Hydroxy quinoline-immobilized bentonite | 293 K | Pb(II) 100–300 mg L−1 | 139.08 | mg g−1 | 71 |
| 303 K | 139.48 | ||||
| 313 K | 141.43 | ||||
| 323 K | 142.94 | ||||
| Montmorillonite–illite | 310 K | Pb(II) 100 mg L−1 | 54.122 | mg g−1 | 252 |
| Pb(II) 150 mg L−1 | 54.517 | ||||
| Pb(II) 200 mg L−1 | 56.768 | ||||
| Mercapto functionalized sepiolite | 289.15 K | Pb(II) 0–180 mg L−1 | 67.62 ± 5.71 | mg g−1 | 79 |
| 299.15 K | 95.50 ± 6.78 | ||||
| 318.15 K | 95.63 ± 6.31 | ||||
| Clay–poly(methoxyethyl)acrylamide composite | 293 K | Pb(II) 100–300 mg L−1 | 3.46 × 10−4 | mol g−1 | 75 |
| 303 K | 3.58 × 10−4 | ||||
| 313 K | 3.85 × 10−4 | ||||
| 323 K | 3.91 × 10−4 | ||||
| Vermiculite | 283 K | Pb(II) 20–200 mg L−1 | 86.96 | mg g−1 | 152 |
| 308 K | 94.61 | ||||
| 333 K | 104.50 | ||||
| 353 K | 109.90 | ||||
| Palygorskite | 293.15 K | pH 5.0 ± 0.1 | 8.4 × 10−5 | mol g−1 | 207 |
| 313.15 K | 1.0 × 10−4 | ||||
| Mordenite | 293 K | Pb(II) 20–100 mg L−1, pH 6.0 | 13.95 | mg g−1 | 186 |
| 303 K | 18.55 | ||||
| 313 K | 21.56 | ||||
| 323 K | 24.48 | ||||
| Natural muscovite | 293 K | Pb(II) 0–100 mg L−1, pH 6.0 | 0.630 | mg g−1 | 43 |
| Francolite | 303 K | 1208.00 | mg g−1 | 206 | |
| 313 K | 543.10 | ||||
| 323 K | 345.13 | ||||
| 333 K | 128.90 | ||||
| Tourmaline | 200 | mg g−1 | 122 | ||
| Natural nontronite | 298 ± 1 K | Pb(II) 0.125–2.500 mmol L−1 | 13.329 | mmol g−1 | 179 |
| 3-Aminopropyltriethoxysilane modified nontronite | 24.799 | ||||
| N-Methylimidazole anchored activated palygorskite | 283 K | 714.29 | mg g−1 | 212 | |
| 298 K | 680.27 | ||||
| 313 K | 666.67 | ||||
| Ca-deficient hydroxyapatite | Pb(II) 10–30 mg L−1 | 19.96 | mg g−1 | 210 | |
| Natural apatite | 0.400 | mmol g−1 | 121 | ||
| Synthetic hydroxyapatite | 0.442 | ||||
| Mg2Al LDH | 303.15 K | Pb(II) 5–23 mg L−1, pH 5.7 ± 0.01 | 66.16 | mg g−1 | 119 |
| 323.15 K | 69.23 | ||||
| 343.15 K | 75.18 | ||||
| Loamy sand soil | 2168.00 | mg kg−1 | 124 | ||
| Amino functionalized MCM-41 | Pb(II) 10–70 mg L−1 | 57.74 | mg g−1 | 116 | |
| Clinoptilolite | 293 K | pH 2.0 | 71.94 | mg g−1 | 183 |
| pH 3.0 | 109.89 | ||||
| pH 4.0 | 117.64 | ||||
| pH 5.0 | 111.11 | ||||
| pH 6.0 | 108.69 | ||||
| pH 7.0 | 92.59 | ||||
| 315–500 μm | 144.92 | ||||
| 500–800 μm | 138.88 | ||||
| 800–1000 μm | 128.20 | ||||
| 1000–1600 μm | 121.95 | ||||
| 100 rpm | 149.25 | ||||
| 150 rpm | 156.25 | ||||
| 175 rpm | 166.66 | ||||
| 225 rpm | 172.41 | ||||
| 298 K | 120.48 | ||||
| 313 K | 169.49 | ||||
| 318 K | 181.81 | ||||
| 343 K | 185.18 | ||||
| Cancrinite-type zeolite | 298 ± 2.1 K | 2.130 | mmol g−1 | 185 | |
| Natural zeolite tuff | 303 K | pH 5.0 | 78.6 | mg g−1 | 83 |
| Activated alumina | 303 K | pH 5.0 | 83.33 | mg g−1 | 111 |
| Hydrous MnO2 | 298 K | pH 3.2–3.5 | 15.78 | mmol g−1 | 96 |
| NiO | 303 K | Pb(II) 100–600 mg L−1 | 909.0 | mg g−1 | 94 |
| Fe3O4 | 298 K | pH 5.5 | 0.14 | mmol g−1 | 101 |
| 313 K | 0.15 | ||||
| 328 K | 0.17 | ||||
| Al2O3-supported iron oxide | 288 K | Pb(II) 0.1–0.8 mM | 0.096 | mmol g−1 | 103 |
| 308 K | 0.110 | ||||
| 318 K | 0.140 | ||||
| TiO2/SiO2 binary mixed oxide | 298 K | pH 6.0 | 97.46 | μmol g−1 | 98 |
| 308 K | 169.77 | ||||
| 325 K | 203.25 | ||||
| Poly(ethyleneimine)–silica gel | pH 5.0–6.0 | 82.64 | mg g−1 | 192 | |
| Glutaraldehyde crosslinked poly(ethyleneimine)–silica gel | 50.76 | ||||
| Thiol-functionalized silica | 298 K | Pb(II) 20–250 mg L−1 | 117.51 ± 5.16 | mg g−1 | 117 |
| N-[3-(Trimethoxysilyl)propyl]-ethylenediamine grafted silica | 293 K | 0.057 | mmol g−1 | 193 | |
| Sand | 293 K | 22.90 ± 0.27 | μmol g−1 | 126 | |
| 303 K | 21.60 ± 0.01 | ||||
| 313 K | 21.50 ± 0.20 | ||||
| 323 K | 26.80 ± 0.39 | ||||
| Iron oxide-immobilized sand | 298 ± 1 K | 2.088 | mg g−1 | 128 | |
| Titanate nanotubes | 70 W | Pb(II) 2–200 mg g−1 | 1000 | mg g−1 | 221 |
| 400 W | 2000 | ||||
| 700 W | 1769 | ||||
| Titanate nanotube | 298 ± 1 K | Pb(II) 0.1–3.0 mmol L−1 | 2.64 | mmol g−1 | 99 |
| Talc | 293 K | 7.994 | 228 | ||
| 303 K | 6.146 | ||||
| 313 K | 6.305 | ||||
| 323 K | 5.359 | ||||
| 333 K | 4.724 | ||||
| 343 K | 4.255 | ||||
| Red mud | 300 | pH 2.0 | 3.44 × 10−4 | mol g−1 | 237 |
| 313 | 3.39 × 10−4 | ||||
| 323 | 3.23 × 10−4 | ||||
| Bagasse fly ash | 303 | 2.50 | mg g−1 | 229 | |
| 313 | 2.30 | ||||
| 323 | 2.10 | ||||
| Vanadium mine tailing | 303 K | 20.73 | mg g−1 | 223 | |
| 313 K | 24.43 | ||||
| 323 K | 46.31 | ||||
| Blast furnace sludge | 298 K | 227.00 | mg g−1 | 238 | |
| 318 K | 161.00 | ||||
| Blast furnace dust | 298 K | 142.00 | |||
| 318 K | 111.00 | ||||
| Blast furnace slag | 298 K | 125.00 | |||
| 318 K | 91.00 | ||||
| Electric furnace slag | 293 K | 33.78 | mg g−1 | 137 | |
| 303 K | 35.84 | ||||
| 313 K | 37.04 | ||||
| Dithiocarbamate chelating resin | 298 K | Cd(II) 1–25 mmol L−1 | 2.15 | mmol g−1 | 136 |
| Se(IV) | |||||
| Iron oxide | 303 K | Se(IV) 10–50 mg L−1, pH ∼4.0 ± 0.5 | 8.47 | mg g−1 | 100 |
| 313 K | 7.30 | ||||
| 323 K | 6.52 | ||||
| Silicon oxide | 303 K | 7.06 | |||
| 313 K | 6.38 | ||||
| 323 K | 4.58 | ||||
| Zn(II) | |||||
| Cankiri bentonite | 296 K | 80.64 | mg g−1 | 39 | |
| Kaolin | 303 K | 2.79 × 10−2 | mmol g−1 | 27 | |
| Ball clay | 4.40 × 10−2 | ||||
| Acid washed kaolin | 303 K | 250 | mg g−1 | 29 | |
| Bentonite | 293 K | pH 3.0 | 15.674 | mg g−1 | 34 |
| pH 5.0 | 21.097 | ||||
| pH 7.0 | 15.060 | ||||
| pH 9.0 | 17.065 | ||||
| Bentonite | 303 K | Zn(II) 10–90 mg L−1 | 68.49 | mg g−1 | 35 |
| Bentonite | Zn(II) 200–700 mg L−1 | 98.04 | mg g−1 | 160 | |
| Phosphate-modified bentonite | 104.17 | ||||
| Sulphate-modified bentonite | 111.11 | ||||
| Bentonite | 293 K | pH 5.0 | 36.63 | mg g−1 | 61 |
Bentonite/iron oxides (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 20 °C) 1 at 20 °C) |
29.67 | ||||
Bentonite/iron oxides (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 85 °C) 1 at 85 °C) |
14.10 | ||||
| Local clay 1 | 298 ± 1 K | Zn(II) 50 mg L−1, pH 5.0 | 1.007 | mmol g−1 | 175 |
| Al/Zr pillared local clay 1 (calcined at 723 K) | 1.119 | ||||
| Al/Ti pillared local clay 1 (calcined at 723 K) | 1.217 | ||||
| Al/Zr pillared local clay 1 (calcined at 873 K) | 1.501 | ||||
| Al/Ti pillared local clay 1 (calcined at 873 K) | 1.547 | ||||
| Local clay 2 | 0.934 | ||||
| Al/Zr pillared local clay 2 (calcined at 723 K) | 1.151 | ||||
| Al/Ti pillared local clay 2 (calcined at 723 K) | 1.578 | ||||
| Al/Zr pillared local clay 2 (calcined at 873 K) | 1.587 | ||||
| Al/Ti pillared local clay 2 (calcined at 873 K) | 1.567 | ||||
| HCl/NaOH-treated bentonite | 295 ± 1 K | Zn(II) 20–200 mg L−1 | 138.17 | mg g−1 | 45 |
| Sodium dodecylsulfate-montmorillonite | Zn(II) 4.5–45 mmol | 202.9 | mmol kg−1 | 171 | |
| Humic acid-immobilized-amine modified polyacrylamide–bentonite composite | 303 K | pH 8.0 | 52.93 | mg g−1 | 168 |
| Tannin immobilised calcined hydrotalcite | 303 K | pH 6.0 | 78.9 | mg g−1 | 118 |
| 313 K | 81.9 | ||||
| 323 K | 91.5 | ||||
| 333 K | 101.7 | ||||
| Natural nontronite | 298 ± 1 K | Zn(II) 0.125–2.5 mmol L−1 | 12.269 | mmol g−1 | 179 |
| 3-Aminopropyltriethoxysilane modified nontronite | 23.098 | ||||
| NaA zeolite | 298 K | Zn(II) 100 mg L−1, pH 6.0 | 118.906 | mg g−1 | 84 |
| NaX zeolite | |||||
| 106.382 | |||||
| Clinoptilolite | 303 K | Zn(II) 100–400 mg L−1 | 133.85 | mg g−1 | 182 |
| Cancrinite-type zeolite | 298 ± 2.1 K | 1.532 | mmol g−1 | 185 | |
| Clinoptilolite | Zn(II) 50–500 mg L−1 | 21.20 | mg g−1 | 184 | |
| NaCl treated clinoptilolite (at 20 °C) | 20.80 | ||||
| NaCl treated clinoptilolite (at 70 °C) | 22.20 | ||||
| HCl treated clinoptilolite | 17.90 | ||||
| Phosphate | 298 ± 1 K | 10.32 | mg g−1 | 209 | |
| Francolite | 303 K | 126.70 | mg g−1 | 206 | |
| Diatomite | 298 K | 27.247 | mg g−1 | 256 | |
| 303 K | 27.777 | ||||
| 313 K | 37.375 | ||||
| MnO2 modified diatomite | 298 K | 16.339 | |||
| 303 K | 14.598 | ||||
| 313 K | 22.026 | ||||
| 323 K | 51.41 | ||||
| 333 K | 38.45 | ||||
| Hydrous MnO2 | 298 K | pH 3.5–4.0 | 0.833 | mmol g−1 | 96 |
| Chinese loess soil | 288 K | 70.80 | mg g−1 | 202 | |
| 298 K | 70.20 | ||||
| 308 K | 75.20 | ||||
| 318 K | 83.20 | ||||
| Sand | 303 ± 2 K | 169 ± 0.2 | μmol g−1 | 127 | |
| Poly(ethyleneimine)–silica gel glutaraldehyde crosslinked poly(ethyleneimine)–silica gel | pH 5.0–6.0 | 52.08 | mg g−1 | 192 | |
| 32.79 | |||||
| Bagasse fly ash | 303 K | 2.34 | mg g−1 | 230 | |
| 313 K | 2.45 | ||||
| 323 K | 2.54 | ||||
| Pit coal fly ash | 277 K | 4.105 | mg g−1 | 233 | |
| 291 K | 5.753 | ||||
| 333 K | 7.655 | ||||
| Powdered marble waste | 298 K | Zn(II) 100–300 mg L−1 | 175.13 | mg g−1 | 227 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layered minerals, kaolinite was shown to have Cr(III) adsorption capacity of 4.43 × 10−5 to 4.98 × 10−5 mol g−1 in the temperature range of 303 to 323 K and 4.40 × 10−5 to 5.83 × 10−5 mol g−1 when the ionic strength was varied from 1 × 10−3 to 1 × 10−1 mol L−1.28 The ionic strength influenced the coulombic interactions between the charged surface and the metal ions through a number of counter ions in the diffuse layer. This affected the charge development on the clay mineral surface and uptake of Cr(III) increased. Adsorption of Cr(III) and Pb(II) on Turkish kaolinite had Langmuir capacities of 21.55 and 18.08 mg g−1 respectively at 293 K.31 Another Turkish kaolinite possessed qm of 31.75 mg g−1 for Pb(II).30
1 layered minerals, kaolinite was shown to have Cr(III) adsorption capacity of 4.43 × 10−5 to 4.98 × 10−5 mol g−1 in the temperature range of 303 to 323 K and 4.40 × 10−5 to 5.83 × 10−5 mol g−1 when the ionic strength was varied from 1 × 10−3 to 1 × 10−1 mol L−1.28 The ionic strength influenced the coulombic interactions between the charged surface and the metal ions through a number of counter ions in the diffuse layer. This affected the charge development on the clay mineral surface and uptake of Cr(III) increased. Adsorption of Cr(III) and Pb(II) on Turkish kaolinite had Langmuir capacities of 21.55 and 18.08 mg g−1 respectively at 293 K.31 Another Turkish kaolinite possessed qm of 31.75 mg g−1 for Pb(II).30For adsorption of a number of cations, Cd(II), Cr(III), Cu(II), Pb(II), Zn(II) and Ni(II) at 303 K, Chantawong et al.27 measured the Langmuir capacity of Thai kaolin to be 6.71 × 10−3, 3.49 × 10−2, 12.00 × 10−3, 3.18 × 10−3 and 2.79 × 10−2 mmol g−1, and of ball clay to be 2.10 × 10−2, 6.90 × 10−2, 2.50 × 10−2, 1.70 × 10−2, 4.40 × 10−2 and 7.00 × 10−3 mmol g−1 respectively. Kaolin was shown to have a preference for Cr(III) than to the softer Lewis acids, Cu(II), Cd(II), Ni(II), Pb(II) and Zn(II). This indicated the main adsorption sites on kaolin to be having Lewis base characteristics. Ballclay, whose main constituent was illite (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type) had excess negative charge due to isomorphous substitution in tetrahedral and octahedral sheets resulting in formation of outer-sphere complexes. The adsorption sites exhibited relatively hard Lewis base characteristics and had highest adsorption for Cr(III). In another work, Langmuir capacity of ball clay (SiO2 53.70%, Al2O3 31.31%, moisture 10.03%) for Cd(II) was found to be 27.27 mg g−1.144 It was suggested that silanol [
1 type) had excess negative charge due to isomorphous substitution in tetrahedral and octahedral sheets resulting in formation of outer-sphere complexes. The adsorption sites exhibited relatively hard Lewis base characteristics and had highest adsorption for Cr(III). In another work, Langmuir capacity of ball clay (SiO2 53.70%, Al2O3 31.31%, moisture 10.03%) for Cd(II) was found to be 27.27 mg g−1.144 It was suggested that silanol [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH,
Si–OH, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si–(OH)2 and
Si–(OH)2 and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) S–(OH)3] and alumina [
S–(OH)3] and alumina [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Al–OH and
Al–OH and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al–(OH)2] groups were involved in adsorption of Cd(II).
Al–(OH)2] groups were involved in adsorption of Cd(II).
Zn(II) was shown to adsorb in the micropores of bentonite through an ion-exchange mechanism at pH 6.64 with a Langmuir capacity of 68.49 mg g−1 at 303 K.35 On the other hand, natural bentonite had Langmuir capacity of 12.38 to 15.92, 6.78 to 16.86 and 15.67 to 17.07 mg g−1 respectively for Cu(II), Co(II) and Zn(II) in a pH range of 3.0 to 9.0 at 293 K.34 The metal cations prefer to bind to the mineral surface at higher solution pH when the surface developed negative charge. In another work, bentonite was shown to have Cu(II) and Cd(II) Langmuir capacities of 12.5 × 10−2 and 6.80 × 10−2 mmol g−1 respectively at 303 K.36 The reported adsorption capacities, however, have wide variance even for similar mineral. Thus, bentonite was shown to have a very large Langmuir capacity for Cu(II) of 909 mg g−1.145 It was proposed that Cu(II) had attached to bentonite via complexation with surface hydroxyl groups and predominantly by cation exchange at the negatively charged surface of the crystallites. Pb(II) Langmuir capacity on natural bentonite increased from 107.0 to 120.0 mg g−1 in the temperature range, 293 to 333 K.146
Langmuir capacity of Na-bentonite has been measured as 9.73 × 10−5 to 1.08 × 10−4 mol g−1 in the temperature range of 293.15 to 333.15 K for Cu(II)37 adsorption by ion exchange with H+/Na+, 17.88 mg g−1 for Cd(II),147 and 2.31 × 10−4 to 3.10 × 10−4 mol g−1 (298 to 338 K) for Pb(II).38
Brazilian bentonite (NT-25) had higher Langmuir capacity of 11.2 and 6.0 mg g−1 for Cd(II) and Mn(II) compared to K10 montmorillonite (Cd(II): 6.3 mg g−1, Mn(II): 4.8 mg g−1) at 298 K.33 Cation-exchange with sites located on (001) basal planes of the clays was shown to be responsible for fast uptake of the cations leading to formation of inner-sphere surface complexes. Chinese bentonite had Langmuir capacity of 1.15 × 10−4 to 1.64 × 10−4 mol g−1 for Pb(II) at temperatures, 293.15 to 343.15 K.32 The interactions were shown to be energy-intensive.
Veli and Alyüz39 showed that higher ionic potential of Zn(II) was responsible for bentonite taking up twice as much Zn(II) (qm: 80.64 mg g−1) than Cu(II) (qm: 44.84 mg g−1).
Surface complexation between Cu(II) and negatively charged SiO− and AlO− on the edges of spent activated clay was shown to be mostly responsible for Cu(II) uptake (qm: 10.9 to 13.2 mg g−1 for pH 5.0 to 6.0; qm: 9.5 to 12.8 mg g−1 at 277 to 323 K).148 In a similar work, Weng et al.149 showed that spent activated clay had Cr(VI) Langmuir capacity of 0.74 to 0.59 mg g−1 (pH: 2.0 to 4.0), 0.74 to 1.42 mg g−1 (277 to 313 K) and 0.95 to 0.47 mg g−1 (ionic strength: 0.005 to 0.1 M). The adverse effect of ionic strength was due partly to the competition between ClO4− and HCrO4− for Si–OH+ and Al–OH+ adsorption sites.
Langmuir capacity of calcined bentonite for Ni(II) increased from 2.875 to 3.893 mg g−1 in the temperature range of 293 to 348 K.150
Natural sepiolite had highest uptake of Cr(III) (0.53 mg g−1) followed by Cd(II) (0.39 mg g−1) and Mn(II) (0.25 mg g−1) at 293 K.151 The interactions were shown to occur through ion-exchange, complex formation and surface adsorption mechanisms. Liu et al.152 reported endothermic interactions of Pb(II) with vermiculite when the Langmuir capacity varied from 86.96 to 109.50 mg g−1 (283 to 353 K). The increased adsorption at higher temperature was attributed to the increase in the number of active sites (Si–OH and Al–OH) as well as to decrease in the adsorption activation energy. In a similar work, vermiculite was shown to have preference for Cr(III) (qm: 46.95 mg g−1) over Cu(II) (qm: 43.67 mg g−1).153 Vermiculite could adsorb metal ions through cation exchange at the planar sites, resulting from the interactions between metal ions and negative permanent charge forming outer-sphere complexes, and also through the formation of inner-sphere complexes through Si–O– and Al–O– groups at the clay particle edges. The comparatively smaller Cr(III) could compete faster for exchange sites than Cu(II).
Interactions of muscovite with As(III), As(V), Cd(II), Cu(II) and Pb(II) yielded Langmuir capacities of 0.33 mg g−1 (b: 0.027 L mg−1), 0.79 mg g−1 (b: 0.172 L mg−1), 0.75 mg g−1 (b: 0.005 L mg−1), 0.62 mg g−1 (b: 0.028 L mg−1) and 0.63 mg g−1 (b: 0.054 L mg−1) respectively at 293 K.43 The mechanism suggested was formation of inner-sphere complexes of As(III), As(V), Cu(II), and Pb(II), and outer-sphere complex of Cd(II).
Kaolinite intercalated with urea followed by dealumination showed better adsorption of Cu(II) (kaolinite 6.2 ± 0.2 mmol g−1, urea-intercalated kaolinite 8.5 ± 0.1 mmol g−1, urea intercalated/delaminated kaolinite 9.6 ± 0.2 mmol g−1) and Pb(II) (kaolinite 6.3 ± 0.2 mmol g−1, urea-intercalated kaolinite 9.6 ± 0.1 mmol g−1, urea intercalated/delaminated kaolinite 9.9 ± 0.2 mmol g−1).154 It is suggested that the inner-sphere interactions particularly at the edge sites of kaolinite dominated the outer-sphere ones. Further, adsorption on intercalated surface is governed by the microenvironment around each site, composed of basic centers, created by hydration.
Acid activated clays have improved Langmuir capacity for Pb(II) over the raw clays155 [qm for raw green clay 25.44 mg g−1, H2SO4 activated green clay 40.75 mg g−1; raw red clay: 17.84 mg g−1; H2SO4 activated red clay 27.15 mg g−1; b: 0.018 to 0.074 L mg−1]. The acid activated clays showed increase in specific surface area, total pore volume, internal porosity and pore size compared to untreated clay. Pb(II) was shown to interact with Si–OH groups on the surface.
Similarly, acid-activated kaolinite (Cd(II) 11.70 mg g−1, Co(II) 12.10 mg g−1, Cu(II) 10.10 mg g−1, Cr(VI) 13.90 mg g−1, Ni(II) 11.90 mg g−1, Pb(II) 12.33 mg g−1, Fe(III) 12.10 mg g−1) and montmorillonite (Cd(II) 33.20 mg g−1, Co(II) 29.70 mg g−1, Cu(II) 32.30 mg g−1, Ni(II) 29.50 mg g−1, Pb(II) 31.35 mg g−1, Fe(III) 30.00 mg g−1) have enhanced Langmuir capacities for a group of metal ions.46,50–52,54,55,57 The process of acid activation could have removed mineral impurities from the surface with partial dissolution of the external layers. This might be a contributing factor in increased adsorption capacity after acid activation (Fig. 7–11).
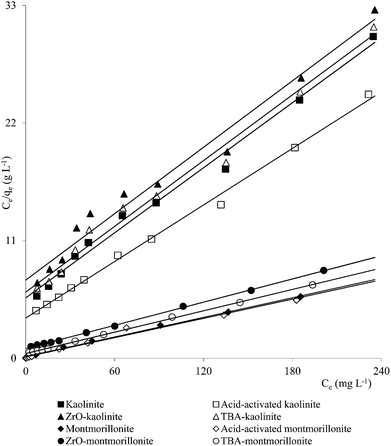 | ||
| Fig. 7 Langmuir plots for Cd(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Cd(II) 10–250 mg L−1, pH 5.5, time 240 min, temperature 303 K). | ||
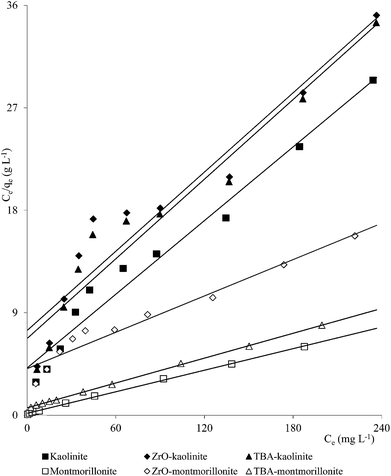 | ||
| Fig. 8 Langmuir plots for Cu(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Cu(II) 10–250 mg L−1, pH 5.7, time 360 min, temperature 303 K). | ||
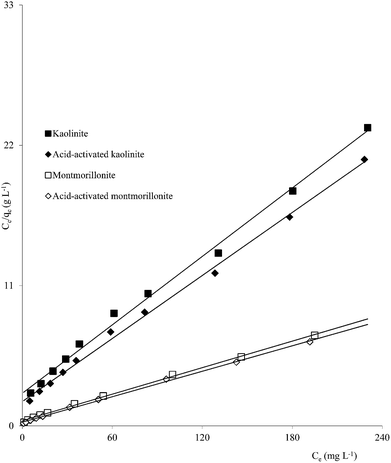 | ||
| Fig. 9 Langmuir plots for Fe(III) adsorption on clay minerals (clay mineral 2.0 g L−1, Fe(III) 10–250 mg L−1, pH 3.0, time 300 min, temperature 303 K). | ||
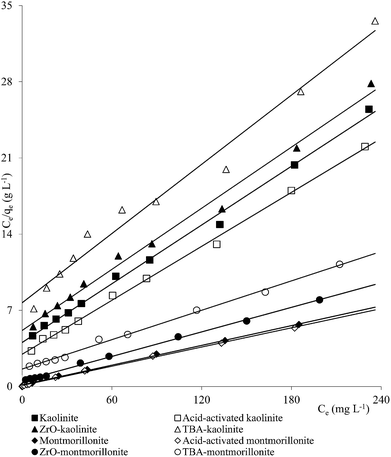 | ||
| Fig. 10 Langmuir plots for Pb(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Pb(II) 10–250 mg L−1, pH 5.7, time 180 min, temperature 303 K). | ||
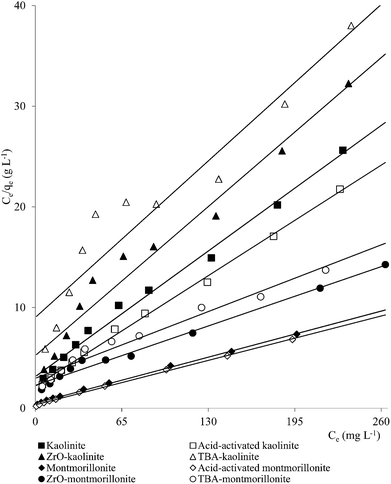 | ||
| Fig. 11 Langmuir plots for Ni(II) adsorption on clay minerals (clay mineral 2.0 g L−1, Ni(II) 10–250 mg L−1, pH 5.7, time 180 min, temperature 303 K). | ||
Bentonite modified with aqueous HCl followed by soaking in NaOH had Langmuir capacity of 138.17 and 202.63 mg g−1 respectively for Co(II) and Zn(II)45 (b for Co(II) 2.39 × 10−3 L mg−1 and Zn(II) 0.61 L mg−1). The cations were held to the clay surface either by proton exchange or surface reaction due to high surface charge developed from the treatments.
A homogeneous mixture of china clay and fly ash had very similar As(III) adsorption capacities at 303 K (0.39 mg g−1, b = 1.590 L mg−1) and 323 K (0.40 mg g−1, b = 5.548 L mg−1).156 It shows that the active surface area for As(III) uptake did not change much in the temperature range.
Na+-montmorillonite showed progressively higher Langmuir capacity for Cd(II) after Al13-pillaring and acid activation (qm: 0.125, 0.139, and 0.163 mmol kg−1, b: 2.582, 2.899 and 5.483 L mmol−1, respectively).64 The b values were in the range of 2.582 to 5.483 L mmol−1. It was shown that intercalation followed by acid treatment increased the basal spacing, specific surface area and total pore volume of the materials resulting in higher uptake of Cd(II). Similarly, Cd(II) was preferably adsorbed on carbon modified aluminum-pillared montmorillonite compared to the unpillared form.157
Al/Ti and Al/Zr-pillared clays, calcined at 723 and 873 K for 2 h, had Zn(II) adsorption capacities of 3.178 to 4.162 mg g−1 at pH 5.0 and temperature 298 ± 1 K.158 The chemisorption process was controlled by positioning and amount of hydroxyl groups in outer regions of the materials, as in lamellae borders or central structures.
Tomul63 reported higher Cd(II) adsorption on Fe-pillared bentonite (0.77 L mmol−1) than Fe/Cr pillared bentonite (0.63 L mmol−1) and Cr pillared bentonite (0.42 L mmol−1). The variations were shown to be due to the differences in pore size distribution.
MgO coated bentonite had Cu(II) Langmuir capacity of 58.44 ± 0.46 mg g−1 (b: 0.22 ± 0.13 L mg−1).59 MgO was shown to act as a buffering stabilizer that minimized the solubility of Cu(II) cations avoiding their redissolution.
Cation-exchange and outer sphere surface complexation had mainly contributed to Pb(II) adsorption (qm: 95.88 mg g−1 at pH 4.0 ± 0.1, b: 9.00 L g−1) on Fe, Mg (hydr)oxides coated bentonite composite in acidic pH.60
Phosphate-immobilized zirconium pillared bentonite had qm of 50.98, 50.07 and 47.81 mg g−1 respectively for Hg(II), Cd(II) and Co(II)159 and it was shown that the adsorption was dependent on the ionic radii of the hydrated metal ions [b for Hg(II): 0.306 L g−1, Cd(II): 0.195 L g−1, Co(II): 0.180 L g−1].
Introduction of inorganic functional groups, sulphate and phosphate, into bentonite resulted in improved Cu(II) and Zn(II) uptake (qm Cu(II): 149.25, 158.73 and 166.69 mg g−1 (b = 4.10 × 10−3, 4.61 × 10−3 and 6.18 × 10−3 L g−1); Zn(II): 98.04, 104.17 and 111.11 mg g−1 (b = 6.59 × 10−3, 7.50 × 10−3 and 8.07 × 10−3 L g−1) for raw, sulphate- and phosphate-modified bentonite respectively).160 The modified clay had enhanced CEC with the anions creating additional adsorption sites on the bentonite surface (Bentonite 95 ± 0.2 mmol per 100 g, sulphate modified bentonite 109 ± 0.2 mmol per 100 g, phosphate modified bentonite 118 ± 0.3 mmol per 100 g). The differences in the degree of hydration of Cu(II) and Zn(II) were reflected in the adsorption capacity values and it was shown that Cu(II) had easier access to the silicate surface than Zn(II).
In a similar work, kaolinite modified with 25% (w/w) aluminium sulphate, was observed to have ∼7 times larger Langmuir capacity (32.2 mg g−1) than the raw kaolinite (4.73 mg g−1) for Pb(II).44 It was proposed that the kaolinite layers were affected by the treatment creating a large number of micro-sized particles and bigger specific surface area. In addition, aluminium sulphate improved the ion exchange capacity of the clay mineral. There was, also consequent variation in Langmuir equilibrium constant [b: 3.00 × 10−2 L mg−1 and 5.04 × 10−2 L mg−1 for kaolinite and modified kaolinite].
Nano-sized iron oxide-coated perlite had Langmuir capacity of 0.39 mg g−1 (b: 44.84 L mg−1) for As(V) oxyanions (H2AsO4− and HAsO42−) at pH 4.0–7.0, the anions being held through coulombic interaction.161
The pillaring did not always lead to enhanced adsorption. Jobstmann and Singh26 found that montmorillonite adsorbed more Cd(II) (qm: 21.9 μM kg−1, b: 0.22 L μM−1) than hydroxy-aluminium-interlayered montmorillonite (qm: 2.5 μM kg−1, b: 0.21 L μM−1) at 293 K. The CEC of montmorillonite decreased by ∼30% after pillaring and this was shown to be the reason for decreased adsorption. Cd(II) was held by electrostatic attraction on raw montmorillonite, but a chemisorptive mechanism was proposed for the pillared montmorillonite.
When kaolinite and montmorillonite were modified by incorporation of ZrO into the interlayer space, the Langmuir capacity of the modified materials actually decreased [kaolinite (mg g−1): Cu(II) 9.20 to 3.00, Cd(II) 6.78 to 5.27, Co(II) 11.20 to 9.00, Pb(II) 11.50 to 9.01, Ni(II) 10.40 to 8.80, Fe(III) 11.20 to 9.70, Cr(VI) 11.60 to 10.90; montmorillonite (mg g−1): Cu(II) 31.80 to 7.10, Cd(II) 30.67 to 36.63, Co(II) 28.60 to 22.30, Pb(II) 31.05 to 31.44, Ni(II) 28.40 to 22.0, Fe(III) 28.90 to 23.80].47,49,53,55,56 It was suggested that there was a net reduction in the number of available adsorption sites due to introduction of ZrO into the clay minerals (Fig. 7. Fig. 8, Fig. 10 and Fig. 11).
Introduction of TBA (tetrabutylammonium) ions into the interlayer space of kaolinite and montmorillonite was shown to result in an actual decrease in the Langmuir capacity with respect to a number of metal ions [kaolinite (mg g−1): Cu(II) 9.20 to 3.20, Cd(II) 6.78 to 6.31, Co(II) 11.20 to 8.40, Pb(II) 11.50 to 5.44, Ni(II) 10.40 to 8.40, Fe(III) 11.20 to 9.30, Cr(VI) 11.60 to 10.60; montmorillonite (mg g−1): Cu(II) 31.80 to 27.30, Cd(II) 30.67 to 43.47, Co(II) 28.60 to 19.70, Pb(II) 31.05 to 30.67, Ni(II) 28.40 to 19.70, Fe(III) 28.90 to 22.60].47,53,55,56,164 The results indicated that introduction of different groups onto clay structure could be utilized to selectively block certain metal ions from being adsorbed on the surface (Fig. 7, Fig. 8, Fig. 10 and Fig. 11).
Organo-clays synthesized from bentonite with alkyl ammonium surfactant [di(hydrogenated tallow) dimethylammonium chloride (∼75%) having 2-propanol (∼14%) and water (∼11%) as impurities] showed that adsorption of Cr(VI) was higher when bentonite was modified with a higher surfactant dose.165 Higher surfactant loading increased the number of cationic adsorption sites and hence, the Langmuir capacity for Cr(VI) [2.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clay–surfactant (w/w) ratio: qm = 8.36 mg g−1, b = 0.0136 L mg−1 (291 K) and qm = 8.51 mg g−1, b = 0.0093 L mg−1 (310 K); 4.75
1 clay–surfactant (w/w) ratio: qm = 8.36 mg g−1, b = 0.0136 L mg−1 (291 K) and qm = 8.51 mg g−1, b = 0.0093 L mg−1 (310 K); 4.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clay–surfactant (w/w) ratio: qm = 13.57 mg g−1, b = 0.1325 L mg−1 (291 K) and qm = 14.64 mg g−1, b = 0.0922 L mg−1 (310 K)]. The probable mechanism involved electrostatic attraction between Cr2O72− anions and positively charged sites developed in the organoclays through surfactant modification.
1 clay–surfactant (w/w) ratio: qm = 13.57 mg g−1, b = 0.1325 L mg−1 (291 K) and qm = 14.64 mg g−1, b = 0.0922 L mg−1 (310 K)]. The probable mechanism involved electrostatic attraction between Cr2O72− anions and positively charged sites developed in the organoclays through surfactant modification.
Montmorillonite modified with poly(hydroxo aluminium) and cetyltrimethyl-ammonium bromide67 had Langmuir capacity of 2.28 × 10−4 mol g−1 for Cr(III) (b = 1.65 × 104 L mol−1). It was proposed that an excess of CTMA cations on the external surface of the modified clay could reverse the surface charge thereby favoring adsorption of Cr(III).
Langmuir capacity for Cr(VI) on smectite and 2-aminomethylpyridine modified smectite was 1.16 ± 0.12 and 1.87 ± 0.11 mmol g−1 at 298 ± 1 K respectively.166 The free pair of electrons on N atoms functioned as good Lewis base and might be responsible for preferential uptake of metal ions by the modified smectites.
Natural and modified smectite (pillared with Al and Zr polyoxycations followed by organofunctionalization with 3-mercaptopropyltrimethoxysilane) adsorbed Cu(II) more than Co(II).167 Langmuir capacities for Co(II) and Cu(II) adsorption were in the order of Zr/3-mercaptopropyl-trimethoxysilane-smectite [Co(II): (25.8 ± 0.2) × 103, Cu(II): (29.4 ± 0.1) × 103 mmol g−1] > Al/3-mercaptopropyl-trimethoxysilane-smectite [Co(II): (23.8 ± 0.1) × 103, Cu(II): (28.3 ± 0.1) × 103 mmol g−1] > natural smectite [Co(II): (13.9 ± 0.1) × 103, Cu(II): (15.7 ± 0.1) × 103 mmol g−1] at 298 ± 1 K. The adsorption capacity of the modified clays depended on the nature of the complex formed on the surface and also on the affinity of the divalent metal cations to the attached ligand. Langmuir capacities showed that the sulfur donor atoms attached to the inorganic backbone had strong affinity for Cu(II).
Humic acid-immobilized-amine-modified polyacrylamide–bentonite composites had Langmuir capacities of 106.21, 96.15 and 52.93 mg g−1 at 303 K for Co(II), Cu(II) and Zn(II) respectively.168 Humic acid had negative charge that enhanced the adsorption of metal cations through electrostatic interactions and/or complexation reactions. The intensity of adsorption (b) also followed the same order as Langmuir capacities i.e. Co(II): 1.32 L mg−1 > Cu(II): 0.21 L mg−1 > Zn(II): 0.03 L mg−1.
Bentonite modified with N-2-hydroxypropyl trimethyl ammonium chloride chitosan yielded qm and b as 22.23 mg g−1 and 0.178 L g−1 for Cd(II) adsorption.169 The adsorption mechanism was based on chelation of Cd(II) with –OH and –NH2 in the surface of modified-bentonite. Cation exchange between Cd(II) and modified-bentonite was also shown to play some role in the adsorption process.
Activated clay with tannin-immobilized on it adsorbed Cr(VI) with Langmuir capacities of 18.34 to 24.09 mg g−1 (b = 0.033 to 0.040 L mg−1) at 300 to 330 K.76 Adsorption mechanism suggested was esterification reaction with phenolic groups of tannin and anionic Cr(VI) species and also through partial reduction of Cr(VI) to Cr(III) followed by ion exchange in acidic medium.
Cr(VI) adsorption on a number of organo-clays [hexadecyl-pyridinium (HDPy)-, hexadecyl-trimethylammonium (HDTMA)-, and benzethonium (BE)-montmorillonite and vermiculite] showed higher Langmuir capacities for the organo-vermiculites (0.28–0.71 mol kg−1) than the organo-montmorillonites (0.23–0.37 mol kg−1) which might be due to larger basal spacing in the former.170 The adsorption mechanism was Cr(VI) anion exchange with Cl−, generated from the organic salts used for modification and present in the structure of organo-clays in the form of ion pairs. It was shown that Cr(VI) anions were held to the interlayer sites more strongly than to the external surface.
Langmuir capacity of dodecylamine modified sodium montmorillonite for Cr(VI)73 was 23.69 mg g−1 (b = 0.109 L mg−1) at pH 2.5 with proposed interactions between the protonated amine and hydrogentetraoxochromate(VI) anion. Hydrogen bonding was also likely between the protonated nitrogen in the primary amine and the OH groups in the clay (NH3+⋯OH) surface.
Sodium dodecylsulfate-modified montmorillonite yielded Langmuir capacities of 254.1 mmol kg−1 (b = 0.46 L mmol−1) and 202.9 mmol kg−1 (b = 0.47 L mmol−1) for Cu(II) and Zn(II) uptake respectively.171 The modified montmorillonite had a much larger surface area than the raw clay, and consequently larger adsorption capacity. Grafting of sodium dodecylsulfate to the clay interlayer space increased the negative charge on the clay surface which might have enhanced the adsorption capacity for cations. Sodium dodecyl sulphate-modified-Fe-pillared montmorillonite had Langmuir capacities of 13.2 to 20.2 mg g−1 for Co(II) and 11.0 to 18.7 mg g−1 for Cu(II).172 The clay-composites had larger surface area but smaller mesopore volume and diameter compared to the host montmorillonite. The decrease in mesopore volume and diameter indicated that some of the pores might have been blocked by the pillaring process.
Bentonite with 8-hydroxy quinoline immobilized on its surface had qm from 51.12 to 56.55 mg g−1 (b = 0.315 to 0.627 L mg−1) for Cu(II)70 and 139.08 to 142.94 mg g−1 (b = 0.103 to 0.307 L mg−1) for Pb(II)71 at 293 to 323 K. Adsorption occurred through silanol –OH groups. Adsorption of Cu(II) on 2,2′-dipyridyl immobilized bentonite followed a similar mechanism72 with Langmuir capacity of 49.44 to 54.07 mg g−1 (b = 4.33 × 10−2 to 0.208 L mg−1) at 293 to 323 K.
Montmorillonite intercalated with dimethyl sulfoxide/3-aminopropyltriethoxysilane (MDS/ASP) and pyridine/3-aminopropyltriethoxysilane (MP/ASP) showed higher Langmuir capacity for Hg(II) [MP/ASP: 1.77 ± 0.12 mmol g−1, MDS/ASP: 1.56 ± 0.12 mmol g−1; raw clay: 1.23 ± 0.12 mmol g−1] at pH 3.0 after an interaction time of 360 min and temperature 298.15 ± 0.2 K.173 Adsorption depended on the nature of the complex formed on the internal and external surface as well as on the affinity of Hg(II) for the organic part.
Adsorption of Ni(II) on montmorillonite K10 and 3-mercaptopropyltrimethoxy-silane-montmorillonite K10 had monolayer capacities of 2.10 mg g−1 (b = 0.010 L mg−1) and 4.73 mg g−1 (b = 0.157 L mg−1) at 298 K.74 The thiol groups on the surface of the modified clays were shown to be responsible for enhanced retention of Ni(II).
Bentonite modified with octadecylbenzyldimethylammonium cations was more efficient in removing As(V) (qm: 1.48 mg g−1, b: 0.11 L mg−1) than As(III) (qm: 0.82 mg g−1, b = 0.17 L mg−1) at 306 ± 1 K.68 It was proposed that As(V) was held through formation of outer-sphere complex via surface anion exchange while inner-sphere complex at Si–O and Al–O groups at particle edges led to As(III) adsorption.
Montmorillonite modified with 3-(2-aminoethylamino)propyltrimethoxysilane had higher Langmuir capacity174 [Cd(II): 12.9, Pb(II): 13.8, Cu(II): 36.0 mmol kg−1] than the ones modified with 3-amino-propyltriethoxysilane [Cd(II): 7.0, Pb(II): 9.7, Cu(II): 28.0 mmol kg−1] and unmodified montmorillonite [Cd(II): 5.3, Pb(II): 7.5, Cu(II): 11.0 mmol kg−1]. The amino-functionalization had created high affinity sites on the clay for holding metal cations. It was shown that the metal cations were bound in monodentate mode to the amino groups, resulting in enhanced binding efficiency.
Ca-montmorillonite modified with humic acid had higher Langmuir capacity for Cr(III) (qm: 15.7 mg g−1, b: 0.300 L mg−1), Cu(III) (qm: 15.3 mg g−1, b: 0.029 L mg−1) and Cd(II) (qm: 14.1 mg g−1, b: 0.030 L mg−1) than the raw clay [Cr(III) qm: 12.4 mg g−1, b: 0.231 L mg−1; Cu(II) qm: 12.6 mg g−1, b: 0.026 L mg−1; Cd(II) qm: 11.9 mg g−1, b: 0.024 L mg−1].66 Humic acid introduced multiple functional groups to the clay surface and the cations complex with these more effectively.
Olu-Owolabi et al.58 reported Cd(II) and Cu(II) Langmuir capacities in the order of goethite/humic acid-modified bentonite (Cd(II): 16.00 mg g−1, Cu(II): 16.42 mg g−1) > humic acid modified bentonite (Cd(II): 15.92 mg g−1, Cu(II): 16.13 mg g−1) > goethite modified bentonite (Cd(II): 12.34 mg g−1, Cu(II): 13.91 mg g−1) > raw clay (Cd(II): 11.26 mg g−1, Cu(II): 11.29 mg g−1) at 323 K. The modified bentonites had higher CEC than the raw clay. The empty d-orbital of Cu(II) was shown to be responsible for giving rise to better complexation between Cu(II) and –OH/COOH groups on the surface rather than the filled d-orbital of Cd(II). It was also suggested that adsorption of Cd(II) was more likely via 5s orbital instead of 4d orbital.
Smectite (S) pillared with (Al13O4(OH)24(H2O)127+, Ti(OC2H5)4, Cr3(OH)44+) and ZrOCl2·8H2O, and organo-functionalized with 3-aminopropyltriethoxysilane (APS)175 had larger Pb(II) Langmuir capacities [9.98 × 103 mg g−1 (SZr/APS), 9.37 × 103 mg g−1 (SCr/APS), 8.91 × 103 mg g−1 (STi/APS), 7.83 × 103 mg g−1 (SAl/APS)] than natural smectite [6.81 × 103 mg g−1] at pH 5.0, temperature 298 ± 0.2 K. Chemical modification was shown to be responsible for making the clay more porous with Pb(II) ions easily entering into the clay structure and interacting with the reactive basic groups anchored in the intercalated smectite.
Very recently, natural and acid-activated sepiolites were functionalized by covalent grafting [3-(2-aminoethylamino)propyl]trimethoxy-silane and were successfully used for removal of Cr(VI) from water.78 The differences in Langmuir adsorption capacities of organo-functionalized sepiolite (qm = 52.99 mg g−1, b = 0.026 L mg−1) and acid-activated organo-functionalized sepiolite (qm = 80.84 mg g−1, b = 0.028 L mg−1) at pH = 2.0 were explained on the basis of protonation of the amine groups and electrostatic attraction of Cr(VI) anionic species.
Pb(II) adsorbed on mercapto-functionalized sepiolite endothermically and the Langmuir capacity increased from 67.62 ± 5.71 to 95.63 ± 6.31 mg g−1 in the temperature range, 289.15 to 318.15 K.79 It was shown that the metal ions were prone to stable complexation with ligands containing soft donor atoms. XPS analysis revealed that one Pb(II) ion reacted with two mercapto groups to form bidentate complex species.
Chitosan immobilized on bentonite showed preferential adsorption for Pb(II) (qm: 26.385 mg g−1, b: 9.974 L mg−1) over Cu(II) (qm: 21.552 mg g−1, b: 0.073 L mg−1) and Ni(II) (qm: 15.823 mg g−1, b: 0.039 L mg−1).176 Pb(II), being highly electronegative, interacted strongly with –OH and –NH2 groups of modified clay compared to Cu(II) and Ni(II).
Bentonite–polyacrylamide composites had higher Langmuir capacity than the raw bentonite for Cu(II)177 [Bentonite qm: 29.43 mg g−1; b: 0.45 L mg−1 at pH 6.2 and qm: 19.89 mg g−1, b: 0.66 L mg−1 at pH 5.0; Bentonite–polyacrylamide composites qm: 32.81 mg g−1, b: 0.19 L mg−1 at pH 6.2 and qm: 19.89 mg g−1, b: 0.46 L mg−1 at pH 5.0]. The increased adsorption capacity was linked to interactions between the lone pair electron of N on the composite surface and empty orbital of Cu(II).
Şölenera et al.75 found that Langmuir capacity for Pb(II) adsorption on clay–poly(methoxyethyl)acrylamide composite varied from 3.46 × 10−4 mol g−1 (b = 4.13 × 103 L mol−1 at 293 K) to 3.91 × 10−4 mol g−1 (b = 8.02 × 103 L mol−1 at 323 K). The presence of olefinic double bonds, carbonyl and amine groups played an active role to attach Pb(II) to the composite.
A novel chitosan–clay (heulandite)–magnetite composite was used for As(V) (qm: 6.50 mg g−1, b: 0.015 L mg−1) and Cu(II) (qm: 14.30 mg g−1, b: 0.176 L mg−1).178 The nano-magnetite and chitosan provided additional adsorption sites on clay.
Pb(II), Mn(II) and Zn(II) from aqueous solution were taken up by natural and 3-aminopropyltriethoxysilane modified nontronite179 with comparatively large adsorption capacities [Pb(II): 13.329 and 24.799 mmol g−1; Mn(II): 13.015 and 23.256 mmol g−1; Zn(II): 12.269 and 23.098 mmol g−1 for nontronite and modified nontronite respectively]. The OH groups anchored in the natural nontronite contributed to the adsorption capacity and the free pair of electrons on the C-atoms in the modified adsorbent definitely boosted the adsorption of metal cations. Besides, the modified adsorbent had larger CEC (126.8 mmol per 100 g) compared to the original (87.0 mmol per 100 g).
Another natural zeolite, clinoptilolite adsorbed Co(II) more than Cu(II), Zn(II) and Mn(II) with Langmuir capacities of 244.13 (Co(II)), 141.12 (Cu(II)), 1333.85 (Zn(II)) and 76.78 (Mn(II)) mmol kg−1.182 It was suggested that the metal cations in the form of hexa aqua complex ions with six surrounding water molecules entered the zeolite channeled and since Co(II) possessed the least diameter among the metal ions, it adsorbed maximum, while Mn(II) had minimum adsorption due to its biggest diameter. Wang et al.83 suggested ion-exchange mechanism for Cu(II) and Pb(II) adsorption on natural zeolite tuff with qm of 23.3 and 78.6 mg g−1 respectively. In another work, Shavandi et al.81 reported Langmuir capacity on clinoptilolite of 1.116 mg g−1 for Fe(III), 0.076 mg g−1 for Mn(II) and 0.015 mg g−1 for Zn(II). An exothermic interaction of natural zeolitic volcanic tuff with Pb(II) was reported by Karatas82 with Langmuir capacities of 16.81, 14.71 and 13.99 mg g−1 for 293 K, 313 K and 333 K respectively for Pb(II) adsorption.
Influence of solution pH, temperature, stirring speed and particle size for Pb(II) adsorption on clinoptilolite was reported by Bektaş and Kara.183 qm varied from 71.94 to 92.59 mg g−1 (pH: 2.0 to 7.0), 120.48 to 185.18 mg g−1 (temperature: 298 to 343 K), 149.25 to 172.41 mg g−1. It was explained that uptake of Pb(II) by clinoptilolite occurred by ion exchange and through physical adsorption when pH was <6.0. Increasing agitation speed reduced the film boundary layer surrounding clinoptilolite particles thus increasing the external film transfer coefficient and hence the rate of uptake.
Clinoptilolite treated with 2 M NaCl at 343 K possessed higher monolayer capacity for Zn(II) than the untreated and HCl treated samples184 [clinoptilolite: 21.1 mg g−1, clinoptilolite treated with 2 M NaCl: 20.8 mg g−1, clinoptilolite treated with 2 M NaCl at 343 K: 22.2 mg g−1, clinoptilolite treated with 0.1 M HCl: 17.9 mg g−1]. Conditioning of clinoptilolite with NaCl enhanced adsorption capacities as NaCl caused an increase in Na+ and a decrease in Ca2+ concentrations in clinoptilolite, leading to an increase of the ratios Na+/K+ and Mg2+/Ca2+. Conditioning with NaCl solution might also have removed fine dust particles from the surface of clinoptilolite crystals.
Qiu and Zheng185 synthesized a cancrinite-type zeolite from fly ash and found that it had Langmuir capacities of 2.13, 2.08, 1.53, 1.24 and 1.15 mmol g−1 for Pb(II), Cu(II), Ni(II), Co(II) and Zn(II) respectively. The small differences were shown to be due to size differences of the hexaaqua complex ions.
Nibou et al.84 reported higher Langmuir capacity of NaA zeolite for Zn(II) than NaX zeolites (NaA: 118.91 mg g−1, NaX: 106.38 mg g−1) at 298 K. The zeolites contained mineral impurities such as Ca, Mg, Fe and K which could interfere in the exchange of Zn(II) with Na(I). In addition, NaA and NaX had small particles with average size of 4 and 3 μm and higher internal surface areas of 280 and 375 m2 g−1 respectively which allowed Zn(II) cations to access and diffuse into the framework structures.
Langmuir capacities of mordenite for Pb(II) varied from 13.95 to 24.48 mg g−1 at 293 to 323 K.186 Mordenite [(Ca, Na2, K2)4 Al8Si40O96·28H2O] with an open structure of 8- and 12-membered rings (2.6 × 5.7 and 6.7 × 7.0 Å, respectively) provided easy access to Pb(II) cations for adsorption inside the channels.
Langmuir capacity of As(III) on Fe(NO3)3 modified zeolite was 40.48 μmol L−1 where Fe–OH groups on the adsorbent surface were proposed as the adsorption sites.187 In a similar study, Javadian et al.89 reported Langmuir capacity of zeolite-based geopolymer for Cd(II) of 26.246 mg g−1. The removal mechanisms included not only adsorption but also reaction with the oxides of iron and aluminum on the surface of zeolite.
A comparative study was reported by Chao and Chen131 for adsorption of Cd(II), Cu(II), Ni(II), Pb(II) and Zn(II) on NaY and hexadecyltrimethylammonium-NaY zeolites. The Langmuir capacities on NaY were in the order Pb(II) (671 mmol kg−1) > Cd(II) (390 mmol kg−1) > Zn(II) (354 mmol kg−1) > Cu(II) (371 mmol kg−1) > Ni(II) (331 mmol kg−1). Ion exchange being the primary mechanism, the adsorption capacities depended on the ion radius with highest adsorption for Pb(II) and least for Ni(II). Adsorption of cations onto hexadecyltrimethylammonium-NaY occurred through both ion exchange and complexation reactions and/or surface precipitation and the adsorption capacities were in the order of Pb(II) (653 mmol kg−1) > Cu(II) (388 mmol kg−1) > Cd(II) (351 mmol kg−1) > Zn(II) (318 mmol kg−1) > Ni(II) (315 mmol kg−1). The presence of the hexadecyltrimethylammonium units on zeolite surface had covered or blocked some of the pores, resulting in slight decreases in the apparent surface area and pore volume. But N atoms in hexadecyltrimethylammonium-ions led to greater adsorption of Cu(II) through complexation reactions. Functional groups containing N atoms could provide nonbonding electrons to coordinate with divalent metals and as Cu(II) possessed relatively higher ability to form such complexes, the adsorption capacity of modified zeolite toward Cu(II) was greater than that of the NaY zeolite.
Nezamzadeh-Ejhieh and Kabiri-Samani87 reported exothermic interactions of Ni(II) with dimethylglyoxime treated clinoptilolite with qm decreasing from 0.96 to 0.13 mmol g−1 (temperature 293 to 333 K). The authors suggested a complex formation between Ni(II) and dimethylglyoxime through the free electron pairs of nitrogen atoms. Langmuir capacity of hexadecyltrimethylammonium-modified clinoptilolite for Cr(VI) decreased several times (5.07 to 0.21 mg g−1) when the temperature of adsorption was raised from 288 to 298 K.188 HCrO4− anions were shown to have bound to ammonium groups.
Langmuir capacities of humic acid immobilized-cetylpyridinium-modified zeolite for Cu(II) increased from 19.8 to 21.5 mg g−1 in the temperature range, 298 to 318 K.88 Adsorption of Cu(II) took place via surface complexation with –COOH and ph–OH groups of immobilized humic acid molecules and also through ion exchange with the exchangeable cations in the internal zeolite channels.
Adsorption of Cd(II) on Al2O3 was proposed to be mostly specific adsorption on surface sites (Al–OH) accompanied by formation of monodenate or bidenate complexes with Al–O− with Langmuir capacity of 89.28 mg g−1 at 303 K.95
γ-Al2O3 [synthesized from NaAlO2 by precipitation with H2SO4 at different temperatures (298 or 353 K) and at different pH (6.0, 7.0 or 8.0) subsequently calcined at 773 K] adsorbed Cd(II), Pb(II) and Zn(II)190 with Langmuir capacities of Pb(II) (9.86 to 13.11 mg g−1) > Cd(II) (5.34 to 8.24 mg g−1) > Zn(II) (5.27 to 7.16 mg g−1). The values were conforming to the decreasing order of the atomic radii: Pb (1.75 Å) > Cd (1.54 Å) > Zn (1.38 Å). Large ionic radius resulted in a weaker repulsion from the positively-charged alumina surface, favoring adsorption on larger pores.
Kailasam et al.191 utilized phosphonic acid modified silica polyamine composite with successive immobilization by Zr(IV) for adsorption of As(V) and measured the Langmuir adsorption capacity of 98.0 mg g−1 (b: 0.016 l g−1 at pH 4.0) and 55.0 (b: 0.018 L g−1 at pH 6.0). Maximum adsorption at pH 4.0 corresponded with the predominant As(V) species, H2AsO4−.
Amino-functionalized mesoporous silica (NH2-MCM-41) showed preferential adsorption of Pb(II) over Cd(II) and Ni(II)116 with qm of 57.74 mg g−1 (b: 0.035 L mg−1), 18.25 mg g−1 (b: 0.253 L mg−1) and 12.36 (b: 0.225 L mg−1) mg g−1 respectively at 298 K.116 Adsorption resulted in complex formation between metal ions and N atoms of NH2 functional groups.
Organo-functionalized silica with 3-aminopropyltrimethoxysilane (APTMS) held Cu(II)115 with Langmuir capacities of 0.485 to 0.850 mmol g−1 for different APTMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica ratio at 303 ± 2 K. Increasing the concentration of amino groups on the surface of silica resulted in an increased affinity towards Cu(II).
silica ratio at 303 ± 2 K. Increasing the concentration of amino groups on the surface of silica resulted in an increased affinity towards Cu(II).
Poly(ethyleneimine) coated silica (silica/PEI) and silica/PEI crosslinked with glutaraldehyde (silica/PEI/GA) had shown large Langmuir capacities for Cd(II), Ni(II), Pb(II) and Zn(II)192 [Pb(II): 50.76, 82.64 mg g−1, Zn(II): 32.79, 52.08 mg g−1, Ni(II): 18.62, 28.25 mg g−1, Cd(II): 29.32, 38.46 mg g−1 for silica/PEI/GA and silica/PEI respectively]. Crosslinking with GA decreased the amount of available amine groups causing a decrease in metal ion uptake.
Thiol-functionalized silica yielded qm of 117.51 ± 5.16 mg g−1 (b: 3.51 ± 0.88 L mg−1) and 33.72 ± 1.23 mg g−1 (b: 2.49 ± 1.25 L mg−1) respectively for Pb(II) and Cd(II) at 298 K.117 Adsorption was through the formation of bidentate and monodentate complexes with thiol groups wherein it was suggested that the metal cations interacted with the highly electronegative S atoms on the surface and shifted the sulfur binding energies to less positive ranges compared to the binding energies of bound thiol groups.
N-[3-(Trimethoxysilyl)propyl]-ethylenediamine grafted silica was shown to be a better adsorbent for Cu(II) and Pb(II) than unmodified silica193 [silica-Pb(II) qm: 0.02 mmol g−1, b: 5.09 L mmol−1; silica-Cu(II) qm: 0.04 mmol g−1, b: 1.75 L mmol−1; modified silica-Pb(II) qm: 0.18 mmol g−1, b: 9.10 L mmol−1; modified silica-Cu(II) qm: 0.26 mmol g−1, b: 12.54 L mmol−1 at 293 K]. Two novel silica gel-based hybrid composites (with amidoxime immobilized via sulfur-containing spacer arm) were effective Hg(II)-adsorbents194 with qm of 0.56 to 0.90 mg g−1 (composite I) and 0.57 to 0.74 mg g−1 (composite II) at 288 to 308 K. In the endothermic interactions, both sulfur and amidoxime present in the adsorbents were suggested to be responsible for chelating Hg(II).
Al2O3-supported iron oxide showed qm for Pb(II) of 0.09 to 0.14 mmol g−1 (b: 17.5 to 48.13 mM−1) at 288 to 318 K. The presence of α-FeOOH as well as large surface area might be responsible for Pb(II) uptake.103
Su et al.96 observed the preferential adsorption of Hydrous MnO2 could adsorb Pb(II), Cd(II) and Zn(II) in the order Pb(II) (1.58 mmol g−1) > Cd(II) (1.25 mmol g−1) > Zn(II) (0.83 mmol g−1) at 298 K, the differences were explained on the basis of the relative softness of the metal cations, electrostatic interaction and inner-sphere complex formation.
Langmuir capacities of ZrO2·nH2O for Cr(VI) increased from 61.0 to 66.0 mg g−1 (b: 0.43 to 0.67 L mg−1 at 298 to 338 K), indicating endothermic interactions.91 The low solubility, high thermal stability, amphoteric character and good resistance to oxidizing agents facilitated the adsorbent to interact with Cr(VI).
Naeem et al.195 reported Co(II) adsorption on NiO by an endothermic process with Langmuir capacities of 8.13 × 10−5 to 8.93 × 10−5 mol g−1 (at 303 to 323 K). The adsorption capacities increased from 6.41 × 10−5 to 17.61 × 10−5 mol g−1 in the pH range of 7.0 to 8.5. It was suggested that increase in hydroxyl ions would dissociate more protons from NiOH surface groups on NiO leaving the surface negatively charged for uptake of Co(II) cations. NiO nanoparticles were shown to possess large Cd(II) and Pb(II) Langmuir capacities of 625.0 mg g−1 (b: 1.734 L mg−1) and 909.0 mg g−1 (b: 9.478 L mg−1) respectively at 303 K.94 It was suggested that, due to smaller hydrated ionic radii, Pb(II) moved faster than Cd(II) to the adsorption sites on NiO nanoparticles.
Langmuir capacities of ZnO followed the trend Hg(II) (qm: 714 mg g−1, b: 4.686 L mg−1) > Cd(II) (qm: 384.0 mg g−1, b: 1.814 L mg−1) > Zn(II) (qm: 357.0 mg g−1, b: 0.753 L mg−1).92 Again, due to smaller hydrated ionic radii, Hg(II) moved faster to the adsorption sites with fewer weakly bound water molecules. The higher electronegativity of Hg(II) (2.0) compared to the others attracted Hg(II) much more strongly to the potential adsorption sites on ZnO. Recently, Venkatesham et al.93 have reported that the larger surface area of nano ZnO might play an important role for Pb(II) adsorption with qm = 26.11 mg g−1 and b = 0.004 L g−1.
Endothermic Langmuir capacity of Co(II) on Mg(OH)2 varied from 95.24 to 125.00 mg g−1 at 298 to 318 K.196 The scattering of small solid flakes on top of one another during adsorption was show to indicate formation of the adsorbed layer on the crystal surface and edges of Mg(OH)2 particles.
Langmuir capacity for As(V) on Fe3O4 nanoparticles (prepared from waste red mud) was 400 μg g−1. The interaction between positive charge of adsorbent surface and H2AsO4− species might be responsible for As(V) removal process.102 Adsorption of Pb(II) on Fe3O4 nanoadsorbents was shown to be electrostatic101 with qm of 0.14 to 0.17 mmol g−1 (b: 24.6 to 33.1 L mmol−1 at 298 to 328 K).
As(V) adsorption on CuFeO4 was influenced by solution pH and the Langmuir capacities varied from 45.66 to 15.06 mg g−1 in the pH range, 3.7 to 11.2 at 300 ± 1 K.197 At lower pH (<7.3), the positively charged copper ferrite surface favoured adsorption of anionic As(V) species. At higher pH, negative charges on both adsorbent and adsorbate, led to electric repulsion and decreased the uptake of As(V).
Bimetal oxide magnetic nanomaterials, MnFe2O4 and CoFe2O4, adsorbed As(III) and As(V) more than Fe3O4 [qm MnFe2O4: As(III) 93.8 mg g−1, As(V) 90.4 mg g−1; CoFe2O4: As(III) 100.3 mg g−1, As(V) 73.8 mg g−1; Fe3O4: As(III) 49.8 mg g−1, As(V) 44.1 mg g−1].104 Adsorption occurred through replacement of OH groups on metal oxides with As(III) and As(V) forming monodentate, bidentate mononuclear and bidentate binuclear complexes. Replacement of Fe2+ with Mn2+ and Co2+ resulted in a significant increase in M–OH species in the magnetic nanomaterials.
Langmuir capacity of glutaraldehyde-3-aminopropyltriethoxysilane modified Fe3O4 nanoparticles for Cu(II) was 0.96 mmol g−1 at pH 4.0 (293 ± 1 K).107 At low solution pH, protonation of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (imine) groups of the adsorbent led to formation of –CH
N– (imine) groups of the adsorbent led to formation of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+–, causing electrostatic repulsion of Cu(II). At pH ≥ 4, the reaction was accompanied by an increase in the number of deprotonated imine groups (–CH
NH+–, causing electrostatic repulsion of Cu(II). At pH ≥ 4, the reaction was accompanied by an increase in the number of deprotonated imine groups (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), thus, increasing the adsorption of Cu(II) cations.
N–), thus, increasing the adsorption of Cu(II) cations.
Modification of Fe3O4 with cetyltrimethylammonium bromide (CTAB) enhanced As(V) adsorption capacity (qm for Fe3O4: 7.59 mg g−1, Fe3O4@CTAB: 23.07 mg g−1).108 The positively charged CTA+ on Fe3O4 surface could attract the negatively charged As(V) anions and CTA–As(V) complex was formed.
Modification of Fe3O4 nanoparticles with carboxymethyl-β-cyclodextrin polymer, Langmuir capacities for Pb(II), Cd(II) and Ni(II) were sufficiently boosted (Pb(II): 20.01 to 64.50 mg g−1, Cd(II): 17.01 to 27.70 mg g−1, Ni(II) 8.83 to 13.20 mg g−1).109 The multiple oxygen containing groups (–OH and –COOH) on the surface in the modified adsorbent supported complex formation with metal cations. Adsorption of Pb(II) on amino-functionalized Fe3O4 magnetic nano-particles yielded Langmuir capacity of 40.10 mg g−1.110
Cd(II) adsorption on SiO2, Fe(OH)3 and an equimolar mixture (0.5 M SiO2–0.5 M Fe(OH)3)198 was higher for the mixture (0.10–0.11 mmol g−1) than either for Fe(OH)3 (0.08–0.09 mmol g−1) or SiO2 (0.03–0.04 mmol g−1) at 288–318 K. The increased adsorption of Cd(II) on the mixed oxide indicated that the surface groups, FeO− and SiO−, were involved in adsorption. When the mixed oxide was prepared by physical mixing or by sequential precipitation, the latter adsorbed more Cd(II) (qm: 9.81 × 10−2 to 10.64 × 10−2 mmol g−1 at 303 to 323 K).199 Formation of ternary complexes (SOH + Cd2+ + H3SiO4− ↔ SH3SiO4−·Cd2+ + OH− where SOH was present on the adsorbent surface) might also be responsible for higher adsorption.
Pb(II) adsorption on nano TiO2–SiO2 showed Langmuir capacities of 97.46 to 203.25 μmol g−1 (b: 0.05 to 0.78 L μmol−1 at 298 to 325 K).98 Preparation of the adsorbent by coprecipitation influenced properties like specific surface area, porosity, acidic sites, etc. of each component with additional influence on adsorption characteristics. Pb(II) may be bonded to adsorbent surface via surface oxygen atoms and proton release.
As(V) removal by a binary mixed oxide of iron and silicon was influenced by solution pH Langmuir capacities of 2.76 × 10−4 to 1.72 × 10−4 mol g−1 (pH 5.0 to 8.0) at 298 K.200 The authors reported higher As(V) adsorption for uncalcined mixed oxide (27.60 × 10−5 mol g−1) than for calcined samples (mixed oxide calcined at 573 K: 25.85 × 10−5 mol g−1 > mixed oxide calcined at 723 K: 9.98 × 10−5 mol g−1 > mixed oxide calcined at 1023 K: × 10−5 mol g−1). It was shown that the small pores on the surface of mixed oxide particles disappear after heat treatment due to sintering resulting in surface area reduction. The authors suggested As(V) adsorption by displacement of the surface hydroxyl groups.
Langmuir capacity for As(V) on Ce doped Fe-oxide was 70.4 mg g−1 (b: 10.91 L mg−1) at 293 K.105 The adsorbent surface could be considered as amphoteric with surface hydroxyl groups (M–OH) playing an important role in As(V) adsorption.
As(V) adsorption on Fe–Ce bimetal oxide201 yielded qm of 2.0 mmol g−1 at 293 K significantly higher than that of Fe oxide (0.35 mmol g−1) and of Ce oxide (0.45 mmol g−1). It was suggested that As(V) anion replaced OH in Fe–OH by forming diprotonated monodentate mononuclear complexes. Ce could break magnetite structure of Fe(II)/Fe(III) system through oxidation of Fe2+ and to activate Fe atoms to form more Fe–OH.
Loess soil had good Langmuir capacities for Cd(II) of 6.68 to 9.37 mg g−1 at 278–318 K,123 which was related to reactions between. Cd(II) and aluminum silicate to form CdSiO3 and a surface complex, CdAl2(SiO4)2. In another work, endothermic interactions of Zn(II) with Chinese loess soil showed larger Langmuir capacities of 70.8 to 83.2 mg g−1 (288–318 K).202
The clay mineral, illite (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layered) constitutes about 70–90% of the loamy sand soil by weight and it has high net negative charge due to isomorphous substitution in the silica tetrahedra that creates ion-exchange sites. The hydroxyl groups, including SiOH and AlOH in the mineral edges were shown to be effective in adsorbing Cr(VI) and Pb(II) with monolayer capacities of 1570 and 2168 mg kg−1 respectively.124
1 layered) constitutes about 70–90% of the loamy sand soil by weight and it has high net negative charge due to isomorphous substitution in the silica tetrahedra that creates ion-exchange sites. The hydroxyl groups, including SiOH and AlOH in the mineral edges were shown to be effective in adsorbing Cr(VI) and Pb(II) with monolayer capacities of 1570 and 2168 mg kg−1 respectively.124
Adsorption of As(V) on lithium/aluminum layered double hydroxide intercalated with chloride (Li/AlLDH-Cl) was shown to have a very large adsorption capacity (qm: 322.58 mmol kg−1) when compared to that of untreated gibbsite (51.55 mmol kg−1).203 Treatment with LiCl brought intercalation of Li cations into the host structure of Al(OH)3 to form the layered double hydroxide. Li cations occupied the vacant octahedral holes within Al(OH)3 and transformed Al(OH)3 layers into active adsorption sites with high affinity for As(V).
Lignite (30–60 g) adsorbed Cu(II) from 4.045 to 2.625 mg g−1 (b: 0.038–0.056 L mg−1).204 Oxygen-containing groups like carboxyl (–COOH), alcoholic (–OH) and carbonyl (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) acyed as the active centers of adsorption.
O) acyed as the active centers of adsorption.
Exothermic adsorption of Cr(VI) on dolomite had qm from 10.01 to 5.67 mg g−1 (b: 0.272 to 0.144 L mg−1 at 293–333 K) with OH− and HCO3− groups playing the active role.205
Langmuir capacities for Pb(II) and Zn(II) on francolite decreased substantially with increasing temperature [Pb(II): 1208.00 to 128.90 mg g−1, Zn(II): 126.70 to 38.45 mg g−1 at 303 to 333 K].206 Adsorption was shown to be due to exchange of Ca2+ with Pb(II) and the larger ionic radius of Pb(II) favoured the ion exchange.
Adsorption of Pb(II) on palygorskite by an endothermic route had Langmuir capacities of 8.4 × 10−5 to 1.0 × 10−4 mol g−1 in the temperature range, 293.15 to 313.15 K.207 It was shown that at higher temperature, a large number of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al–OH and
Al–OH and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) XNa sites participated in adsorption.
XNa sites participated in adsorption.
Langmuir capacities of laterite for As(V) varied from 0.15 to 0.14 mg g−1 (pH 5.5 to 7.0), 0.13 to 0.28 mg g−1 (293 to 315 K) and 0.51 to 0.31 mg g−1 (ionic strength 0.01 to 0.00 mol L−1).208 The oxides present on the surface were shown to be responsible for As(V) adsorption. A low-grade phosphate was shown to have larger adsorption capacity for Zn(II) than Cd(II) [Zn(II): qm 10.32 mg g−1, b: 0.23 L mg−1; Cd(II): qm 7.54 mg g−1, b: 0.20 L mg−1] at 298 ± 1 K.209 The results might have been affected by the larger size of Cd(II) compared to that of Zn(II).
Langmuir capacity of synthetic hydroxy apatite for Pb(II) was found to be marginally higher (0.44 mmol g−1) than that of natural apatite (0.40 mmol g−1).121 Zhu et al.210 used Ca-deficient hydroxyapatite for Cd(II) and Pb(II) removal with Langmuir capacities of 23.04 and 19.93 mg g−1 respectively. Nano hydroxyapatite adsorbed Pb(II) (1000.00 mg g−1) much more than Cd(II) (142.857 mg g−1) and Ni(II) (40.00 mg g−1). Hard Lewis bases like OH− and PO43− in the nano hydroxyapatite had higher affinity for Pb(II) (borderline hard Lewis acid) than for Cd(II) and Ni(II) (soft Lewis acids).
Tourmaline had Pb(II) Langmuir capacity of 200 mg g−1 (b: 0.01 L mg−1).122 Tourmaline acted as an electric dipole with small, separate electric fields and Pb(II) ions were held through electrostatic attraction. By crushing tourmaline into small particles, the cations in tourmaline (e.g. Mg2+, Ca2+, Na+) became exposed along different directions of the crystal fracture and these were lost to water in a tourmaline suspension creating negative tourmaline surface ready for Pb(II).
Very high adsorption capacity for Cd(II) (qm = 434.78 mg g−1, b = 0.077 L mg−1) and Cu(II) (qm = 357.14 mg g−1, b = 0.094) was recently reported for goethite mineral.113 Natural minerals (hematite, magnetite and goethite) had also shown appreciable adsorption of As(III) and As(V) from water.211 The adsorption capacities were: hematite [As(III): (9.3 ± 0.2) × 10−6, As(V): (2.9 ± 0.5) × 10−5 mol m−2]; goethite [As(III): (2.5 ± 0.1) × 10−6, As(V): (3.0 ± 0.2) × 10−6 mol m−2]; magnetite [As(III): (3.1 ± 0.1) × 10−6, As(V): (3.8 ± 0.2) × 10−6 mol m−2]. Adsorption of Cd(II) on boehmite and goethite was shown to have qm of 3.55 and 5.18 mg g−1 respectively.114 A specific adsorption mechanism was proposed for the process.
Exothermic interactions between N-methylimidazole anchored activated palygorskite and Pb(II) yielded Langmuir capacities of 714.29 to 666.67 mg g−1 (b: 0.33 to 0.109 L mg−1 at 283–313 K).212
The presence of –NH2 and –OH groups in hydroxyapatite–chitosan composite favoured adsorption of Pb(II) (qm: 12.04 mg g−1, b: 0.572 L mg−1), Co(II) (qm: 10.63 mg g−1, b: 0.349 L mg−1) and Ni(II) (qm: 8.54 mg g−1, b: 0.211 L mg−1).213 The hydrated ionic radii of the metal ions (Pb(II) < Co(II) < Ni(II)) ensured that smaller hydrated ions possessing higher ionic mobility and rate of diffusion showed higher adsorption.
Adsorption of Cr(III) and Fe(III) onto chitosan–attapulgite composites was endothermic and the Langmuir capacities increased from 27.03 to 65.36 mg g−1 and 36.76 to 62.50 mg g−1 respectively (298–318 K).132 At higher temperature, enlargement of pore volume and surface area along with increased mobility of the ions led to increased adsorption.
Polyaniline–attapulgite composite yielded Langmuir capacities for Hg(II) of 909.1, 813.1 and 781.3 mg g−1 at ionic strengths of (NaNO3) 0.01, 0.10 and 1.00 mol L−1 respectively.214 The adsorption capacity of Hg(II) by bare attapulgite and pure polyaniline were <5 mg g−1 and <600 mg g−1 and it was shown that the fibrous attapulgite acted as a template in the coating of polyaniline, successfully preventing its aggregation and achieved a significantly enlarged surface area. The combined effects led to enhanced Hg(II) removal.
Rectorites had different adsorption capacities for Cr(VI) when modified with dodecyl benzyl dimethyl ammonium chloride (0.97 mg g−1), hexadecyl trimethyl ammonium bromide (2.39 mg g−1) and octadecyl trimethyl ammonium bromide (3.57 mg g−1).215 The differences were explained on the basis of changes in d-spacing following organic functionalization.
Endothermic adsorption of Pb(II) on beach sand yielded qm of 22.90 ± 0.27 to 26.80 ± 0.39 μmol g−1 (293 to 323 K).126 The minerals, calcite, quartz and aragonite in the form of carbonates and oxides in the beach sand had negative sites that provided the affinity for Pb(II) cations. The same group of authors reported Langmuir capacity of the beach sand for Zn(II) of 16.9 ± 0.2 μmol g−1.127
Iron oxide-coated sand had a larger Langmuir adsorption capacity for As(III) (28.57 μg g−1) in comparison to the uncoated one (5.63 μg g−1) at 300 ± 2 K.216 The iron oxide-coated sand adsorbed more due to formation of ferrichydroxide in the aqueous solution responsible for coprecipitation of As(III) on the surface of the adsorbent. Li et al.76 used iron oxide nano-particle-immobilized-sand as adsorbent for Cd(II), Cu(II) and Pb(II) with Langmuir capacities of 0.53, 1.26 and 2.09 mg g−1 respectively. While ion-exchange was a common process for all the cations, adsorption of Cu(II) was also through inner-sphere surface complexation, and electrostatic attraction was shown to be important in case of Cd(II) and Pb(II). H2SO4 treated river sand preferred lower temperature to adsorb Cr(VI) as Langmuir capacities varied from 1.19 mg g−1 (298 K) to 1.10 mg g−1 (308 K).129
During the last few years, nano-scale adsorbents played an active role in metal ion removal scenario. In one such study, Nanoscale zero-valent iron yielded a Langmuir capacity of 135.0 mg g−1 at 298 K for As(III).217 The reactive surface sites were suggested to be stable or metastable iron(II), mixed iron(II)/(III), or iron(III) oxide, hydroxide, or oxyhydroxide. Initially amorphous Fe(II)/(III) and magnetite (or maghemite) were the sites for adsorption, but as the adsorbent corroded over longer periods, more crystalline magnetite and lepidocrocite products were generated for further adsorption of As(III). In addition, As(III) near or in contact with the corroding surface was oxidized to As(V), which was adsorbed by an inner-sphere mechanism. Boparai et al.218 reported qm of nano zero-valent iron for Cd(II) of 714.30 mg g−1 (b: 0.07 L mg−1 at 285 K). The standard redox potential of Cd(II) (−0.40 V, 298 K) was very close to that of zero-valent iron (−0.41 V, 298 K) and thus, adsorption was a predominant process.
Adsorption of Cr(VI) on zero-valent iron, Fe–Ni bimetallic nanoparticles and Fe–Ni bimetallic-montmorillonite nanocomposites Kadu and Chikate62 showed adsorption capacities of 76.92 mg g−1 (b = 2.17 L mg−1), 100.00 mg g−1 (b = 2.00 L mg−1) and 100.00 mg g−1 (b = 3.33 L mg−1) respectively. The lowest adsorption capacity of zero-valent iron was explained on the basis of surface passivation whereas greater uptake of metal–clay composite might be due to synergistic effect of nanoparticles and clay matrix owing to proper dispersion.
It was, however, observed that adsorption of Cr(VI) on magnetite nanoparticles (qm: 20.13 mg g−1) was more than montmorillonite-supported magnetite nanoparticles (qm: 13.86 mg g−1) at 298 ± 2 K.219 The same group of authors220 used magnetite nanoparticle (Mag), diatomite supported magnetite (MagDt-H) and commercial magnetite (MicroMag) for Cr(VI) adsorption. The magnetite nanoparticles showed the highest monolayer capacity (20.16 mg g−1). Cr(VI) uptake was a physico-chemical process, including an electrostatic attraction followed by a redox process in which Cr(VI) was reduced to Cr(III) and fixed into iron oxide.
Chen et al.221 reported the use of titanate nanotubes (prepared with microwave intensities of 40, 400 and 700 W) for Pb(II) removal with qm of 1000, 2000 and 1769 mg g−1 respectively at 298 K. Exchange with Na+ and binding with O-atoms on the adsorbent surface accounted for most Pb(II) removal. In another work, titanate nanotubes were shown to have Langmuir capacities in the order: Pb(II) (2.64 mmol g−1) > Cd(II) (2.13 mmol g−1) > Cu(II) (1.92 mmol g−1) > Cr(III) (1.37 mmol g−1).99 The differences were explained on the basis of hydration energy of the cations.
Hg(II) adsorption on hybrid mesoporous aluminosilicate sieve (prepared with fly ash and impregnated with zeolite A precursors) yielded Langmuir capacity of 20.66 mg g−1 where the electrostatic interactions and ion-exchange mechanism played a role.80
Polymetallic sea nodules preferably adsorbed As(V) (qm: 2.85 to 10.2 mg g−1) than As(III) (qm: 0.69 to 0.31 mg g−1) (As(III), As(V) 0.2 to 0.1 mg L−1).222 As(III)–sea nodule surface formed inner sphere complex, while As(V) partially formed inner and partially outer sphere complexes.
Adsorbent prepared from mine tailings had qm of 20.73 to 46.31 mg g−1, 16.23 to 28.27 mg g−1, 5.36 to 14.96 mg g−1, 3.64 to 5.25 mg g−1 and 3.52 to 8.83 mg g−1 respectively (303 to 313 K) for Pb(II), Cr(III), Cu(II), Ni(II) and Cd(II).223 The high tendency of Pb(II) for specific adsorption was due to its high metal electronegativity. Cu(II) and Ni(II) have similar electronegativities and ion radii, but Cu(II) was adsorbed more since it was bound mainly as the hydroxy complex. On the other hand, large ionic radius of Cd(II) worked against its entry into the interlayers and steric hindrance and lower electrostatic attraction limited its complexation
The influence of soil particle size (sand, coarse silt, fine silt and clay) on Cd(II) adsorption was reported by Roth et al.224 The Langmuir capacities were in the range of 0.19 to 0.50 mg g−1. Generally the granulometric part of the soil had better adsorption, with or without organic matter. The authors suggested that organic matter widely influences the isotherms. Coarse and fine silt, and also clay have numerous adsorption sites. Humic substances make the surface more negative and enhance adsorption of metal cations.
Adsorption on a geopolymer at pH 4.0 was in the order of Pb(II) (147.06 mg g−1) > Cd(II) (67.57 mg g−1) > Cu(II) (48.78 mg g−1) > Cr(III) (19.94 mg g−1).225 The differences were explained on the basis of ion mobility, energy of hydration, etc.
Industrial by-products have also been investigated for uptake of metal ions from aqueous system. In one such work, interaction of Cu(II) with limestone yielded qm of 0.29 mg g−1 at pH 7.0 (298 K).226 Adsorption of Cu(II) was suggested to take place via H-bonding and surface precipitation of the metal cations as colloidal insoluble hydroxide, Cu(OH)2, forming successive layers on the adsorbent surface.
By using marble wastes as adsorbent for Zn(II), Gazy and Gad227 obtained Langmuir capacity of 175.13 mg g−1. Zn(II) ions were chelated to O-atoms on the marble surface together with ion exchange between Zn(II) and Ca(II). Similarly, talc was shown to have Langmuir capacities for Pb(II) of 7.99 to 4.26 mg g−1 in the temperature range, 293 to 343 K.228
Bagasse fly ash (from sugar industry) yielded exothermic Langmuir capacities of Cr(VI): 2.50 to 2.10 mg g−1 and Pb(II): 4.35 to 4.25 mg g−1 (303 to 323 K).229 However, the interactions were endothermic for Cu(II): 2.26 to 2.36 mg g−1 and Zn(II) 2.34 to 2.54 mg g−1,230 and for Cd(II): 1.24 to 2.00 mg g−1 and Ni(II): 1.12 to 1.70 mg g−1 (ref. 231) in the same temperature range. The presence of oxides (SiO2, Al2O3, CaO, Fe2O3, MgO) and the minerals, mullite, haematite, kaolinite, α-quartz, γ-alumina, and geolite on fly ash helped adsorption. In another study, fly ash (from thermal power plant) showed exothermic adsorption of Cu(II) (qm: 5.71 to 5.56 mg g−1, b: 3.97 × 10−3 to 4.35 × 10−3 L mg−1).232 Pit coal fly ash adsorbed Cu(II) and Zn(II) with Langmuir capacities of 4.59 to 7.61 mg g−1 and 4.11 to 7.65 mg g−1, respectively (277 to 333 K).233
Adsorption of Cd(II) on two fly ash samples (Afsin-Elbistan and Seyitomer) was pH dependent and qm varied from 0.08 to 0.29 and 0.01 to 0.23 mg g−1 respectively (pH 3.0 to 7.0).234 The differences were explained on the basis of different chemical composition of the two samples. Adsorption of Cr(VI) and Hg(II) on fly ash (FA) (from thermal power plant) and modified fly ash (impregnated with 0.1 M Al(NO3)3, IFAAl and 0.1 M Fe(Cl)3, IFAFe) had Langmuir capacities of FA 1.38, IFAAl 1.82, IFAFe 1.67 mg g−1 for Cr(VI) and FA 11.00, IFAAl 12.50, IFAFe 13.40 mg g−1 for Hg(II).235 The modified adsorbents were shown to have developed new active sites through electrostatic interactions among Al(OH)3, Fe(OH)3, and SiO2 leading to improved adsorption.
Fly ash activated with bentonite clay yielded Langmuir capacity of 25.907 mg g−1 for Cd(II), which was held to the surface mainly by chemical bonding with ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO− and
SiO− and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) AlO− groups.236
AlO− groups.236
Use of red mud (from aluminium industry) for Cr(VI) and Pb(II) removal yielded qm of 4.36 × 10−4 to 4.05 × 10−4 mol g−1 (b: 0.40 × 10−3 to 0.316 × 10−3 L mol−1) and 3.44 × 10−4 mol g−1 to 3.23 × 10−4 mol g−1 (b: 15.90 × 10−3 to 2.05 × 10−3 L mol−1) (303 to 323 K) respectively.237 Adsorption of As(III) and As(V) on activated red mud with a probable ligand-based mechanism yielded qm of 7.22 and 102.00 mmol g−1 respectively.138
Langmuir capacities of electric furnace slag for Pb(II) and Cr(VI) increased from 33.78 to 37.04 mg g−1 and 32.68 to 39.22 mg g−1 respectively.137 The metal uptake was through calcium-ferrite, CaO·Fe2O3, CaO·2Fe2O3, 4CaO·7Fe2O3 and calcium-manganese-silicate, (Ca, Mn)2SiO4 and CaO·MgO·SiO2 phases.
Steel plant wastes had large qm for Pb(II): furnace sludge (227 and 161 mg g−1) > blast furnace dust (142 and 111 mg g−1) > blast furnace slag (125 and 91 mg g−1) at 298 and 318 K respectively.238 Blast furnace sludge and dust contained iron oxides while silicates of calcium and aluminum and quartz were the major components of the slag.
Low-cost adsorbents, clarified sludge (steel industry), activated alumina, fuller's earth and fly ash (from thermal power plant) could adsorb Cr(VI)239 with Langmuir capacities, clarified sludge 26.51 mg g−1; activated alumina 25.57 mg g−1, fuller's earth 23.58 mg g−1 and fly ash 23.86 mg g−1 at 303 ± 2 K. Use of clarified sludge for Cd(II) adsorption yielded Langmuir capacity of 36.23 mg g−1 at 303 ± 0.5 K.240 Langmuir capacities of Cu(II) on two sewage sludge ash samples140 were 3.28 and 4.14 mg g−1.241 Presence of CaSiO2, CaOFe2O3, CaOSiO2 phases and traces of CaO·MgO·SiO2 with Al2O3 and FeO might be responsible for the adsorption process. Waste material from boron enrichment process with ulexite, NaCaB5O9·8H2O, as the main phase adsorbed Cd(II) (qm: 122.22 mg g−1) and Zn(II) (qm: 107.65 mg g−1).
Adsorption of Co(II) on two ion exchange resins (IRN77 and SKN 1) yielded Langmuir capacities of 86.17 and 69.44 mg g−1 at 298 ± 1 K.135 In a recent work, dithiocarbamate chelating resin was found to adsorb Hg(II) (qm = 2.45 mmol g−1) more than Cd(II) (qm = 2.15 mmol g−1) and Pb(II) (qm = 1.24 mmol g−1).136 It was suggested that the hydrophilic character of the resin played an important role in the uptake of metal ions.
2.3. Dubinin–Radushkevich isotherm
Dubinin–Radushkevich (DR) isotherm,242 which is based on the Polanyi theory,243 has been found useful in computing energy of molecular adsorption. The DR isotherm predicts heterogeneity of the adsorption energies or adsorption space. This isotherm is applicable from trace to saturation values and the adsorbate properties do not differ from the corresponding bulk phase except for its lower energy.244The isotherm has the following form,
qe = qmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−βε2) exp(−βε2)
| (5) |
The linear form will be,
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qmax − βε2 qmax − βε2
| (6) |
ε = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(1 + 1/Ce) ln(1 + 1/Ce)
| (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe vs. ε2.
qe vs. ε2.
The Dubinin–Radushkevich coefficient, β, is related to the mean free energy, E (kJ mol−1) of adsorption per mol of the adsorbate and is equivalent to the work done in transferring one mol of adsorbate molecules from the bulk of the solution to the surface of the adsorbent. The relationship between the two is,245
| E = 1/(2β)1/2 | (8) |
It has been shown that if the magnitude of E is between 8 and 16 kJ mol−1, strong adsorption results from ion-exchange interactions, but if E < 8 kJ mol−1, the adsorption is largely physical in nature.246–248 Most of the experimental works have been interpreted on this basis.
Li et al.37 reported endothermic interaction of Cu(II) on a local bentonite with DR capacities 2.57 × 10−4 to 3.24 × 10−4 mol g−1 (293.15 to 333.15 K). Adsorption was ascribed to ion exchange of H+/Na+ with Cu(II) [β: 1.53 × 10−2 mol2 kJ−2 (293.15 K), 1.60 × 10−2 mol2 kJ−2 (313.15 K), 1.56 × 10−2 mol2 kJ−2 (333.15 K) ]. The Celtek clay had DR capacities of 5.83 × 10−5 and 5.27 × 10−5 mol g−1 respectively for Cr(III) and Pb(II) at 293 K.31 However, adsorption was very weak due to very small adsorption energy [(Pb(II)) 0.10 kJ mol−1 and (Cr(III)) 0.09 kJ mol−1].
DR capacities of natural bentonite for Zn(II), Cu(II) and Co(II) was influenced by solution pH and increased from 9.28 to 17.29 mg g−1, 7.04 to 12.93 mg g−1 and 4.24 to 13.09 mg g−1, respectively (pH: 3.0 to 9.0).34 Increased adsorption at higher pH was due to bentonite surface becoming negative above the point of zero charge (pzc) of 6.35. The mean adsorption energies were 9.71, 3.72 and 0.74 kJ mol−1 respectively for Zn(II), Cu(II) and Co(II) at pH 9.0 (293 K). Thus, the mechanism of adsorption may vary from ion-exchange to weak physical adsorption from Zn(II) to Co(II). Veli and Alyüz39 observed much lower adsorption energy of natural bentonite for Zn(II) (1.25 kJ mol−1) and Cu(II) (0.53 kJ mol−1) but large adsorption capacity [Zn(II): 7266.07 mg g−1, Cu(II): 2997.73 mg g−1].
Yang et al.38 reported Pb(II) uptake on Na-bentonite with DR capacities of 2.14 × 10−3 to 2.67 × 10−3 mol g−1 (298 to 338 K). Adsorption on Na-bentonite surface was attributed to chemical adsorption (E = 10.01 to 13.06 kJ mol−1) and ion exchange was also contributed in the process.
Turkish vermiculite–Cr(VI) interactions had DR capacity of 1.6 × 10−3 mol g−1 and mean adsorption energy of 5.9 kJ mol−1.250 It was shown that adsorption was controlled by Si–OH and Al–OH groups on the vermiculite surface.
Pb(II) cations were taken up by montmorillonite–illite through an ion exchange mechanism with large DR capacities of 39.79 to 50.09 mol g−1 (Pb(II) 100–200 mg L−1).251 Pb(II) cations were shown to be preferentially adsorbed on silanol groups (Si–OH) present on the clay. However, montmorillonite alone had a much lower Cu(II) removal capacity of 13.3844 mg g−1.252
Adsorption of Pb(II) on Fe, Mg (hydr)oxide coated bentonite composite had free energy of 8.45 kJ mol−1 and DR capacity of 108.82 mg g−1.60
Sodium dodecylsulfate-montmorillonite yielded DR capacities of 83.0 mmol g−1 and 31.9 mmol g−1 for Cu(II) and Zn(II) respectively.171 Introduction of sodium dodecylsulfate to the interlayer space of montmorillonite was shown to increase the negative charge at clay surface favouring adsorption of the metal cations.
Anirudhan and Suchithra168 reported that DR coefficient, β, of humic acid-immobilized amine-modified polyacrylamide–bentonite composite for Co(II), Cu(II) and Zn(II) adsorption was 8.67, 8.51 and 7.96 kJ mol−1 respectively. The comparatively high values indicate adsorption to be taking place through ion exchange.
Chitosan immobilized-bentonite adsorbed Pb(II) (qmax: 28.86 mmol g−1), Cu(II) (qmax: 18.80 mmol g−1) and Ni(II) (qmax: 13.27 mmol g−1).176 Th cations were bound through –NH2 and –OH groups and the large Pb(II) DR capacity was due to its higher electronegativity.
In another work, Li et al.76 reported DR capacities of tannin-immobilized activated clay for Cr(VI) was in the range of 11.53 to 14.35 mol g−1 (300 to 330 K). The porousness of activated clay could make it absorb a small part of Cr(VI), partly Cr in the form of anionic species was adsorbed through esterification reaction with phenolic groups of tannin and the adsorption of reduced Cr(III) ions occurred through the ion exchange reaction in acid solution.
Clay–poly(methoxyethyl)acrylamide composite adsorbed Pb(II) in an endothermic process yielding DR capacities of 8.51 × 10−4 to 9.47 × 10−4 mol g−1 (293 to 323 K), the participating groups in adsorption being olefinic double bonds, carbonyl and amine groups present in the composite.75
Cu(II) adsorption on bentonite and bentonite–polyacrylamide composite had DR capacities of 2.52 and 3.68 mmol g−1 at pH 6.2 and 0.45 and 1.06 mmol g−1 at pH 5.0 with DR β of 9.70 and 13.50 kJ mol−1, respectively.177 The interactions were quite strong and were likely to involve coordination between the lone-pair electrons of N and the empty orbital of Cu(II).
DR capacities of 8-hydroxy quinoline-immobilized bentonite for Pb(II)71 and Cu(II)70 were 218.82 to 173.95 mg g−1 and 201.89 to 222.37 mg g−1 respectively (293 to 323 K). Strong Cu(II) uptake on 2,2′-dipyridyl-immobilized bentonite (93.94 to 76.16 mg g−1, 293 to 323 K)72 was supported by large DR adsorption energy of 12.12 to 20.71 kJ mol−1.
Hu et al.67 reported an adsorption energy of 13.9 kJ mol−1 for Cr(V) adsorption on poly(hydroxo aluminium) and cetyl trimethylammonium bromide modified montmorillonite. Marjanović et al.77 showed that DR capacities of (3-mercaptopropyl)trimethoxysilane functionalized natural and acid-activated sepiolite for Cr(VI) were 2.68 to 1.41 mg g−1 and 5.47 to 5.34 mg g−1 (pH 2.0 to 4.5) respectively. Better functionalization was achieved in case of the acid-activated sepiolite that increased the number of silanol groups and resulted in higher adsorption capacity. Cr(VI) anions were adsorbed mostly through an electrostatic mechanism involving protonated mercapto groups on the functionalized sepiolite surface. The authors also suggested that Cr(VI) might have been reduced to Cr(III) by mercapto groups, which were then attracted to the sulfonate groups (–SO2O−), generated by oxidation of the mercapto groups. The adsorption free energy change was only 0.02 and 0.59 kJ mol−1 respectively for the two adsorbents and therefore, Cr(VI) anions were held with weak physical forces only. In a recent report, the same authors78 achieved DR capacities for Cr(VI) of 25.82 and 38.72 mg g−1, respectively with [3-(2-aminoethylamino)propyl]trimethoxy-silane grafted natural and acid-activated sepiolites.
On the other hand, interactions of Pb(II) with mercapto functionalized sepiolite were chemical in nature with adsorption free energy of 14.12 to 15.88 kJ mol−1 and DR capacities of 71.34 ± 4.59 to 105.08 ± 3.11 mg g−1 in the temperature range of 289.15 to 318.15 K.79 Pb(II) cations were shown to directly interact with mercapto groups forming stable inner- and outer-sphere complexes.
Clinoptilolite adsorbed Co(II) (qmax: 0.50 mmol kg−1), Zn(II) (0.29 mmol kg−1), Cu(II) (0.22 mmol kg−1) and Mn(II) (0.15 mmol kg−1).182 The metal ions were adsorbed in the form of their hexaaqua complexes and the largest Mn(II) showed minimum adsorption and the smallest Co(II) had maximum. In a similar work, Argun85 observed DR capacities of clinoptilolite for Ni(II) in the range of 1.81 to 1.58 mg g−1 (293 to 313 K).
Mordenite with low Si/Al ratio (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) removed Pb(II) with DR capacities of 60.38 to 104.13 mg g−1 (293 to 323 K) and adsorption free energies of 9.57 to 9.95 kJ mol−1.186
1) removed Pb(II) with DR capacities of 60.38 to 104.13 mg g−1 (293 to 323 K) and adsorption free energies of 9.57 to 9.95 kJ mol−1.186
Endothermic adsorption of Cu(II) on humic acid-immobilized cetylpyridinium bromide-modified zeolite yielded DR capacities of 27.1 to 28.0 mg g−1 (298 to 318 K), and adsorption energy of 24.6 to 29.3 kJ mol−1, which indicated strong chemical binding.88
Javadian et al.89 reported physisorptive interactions between Cd(II) and zeolite-based geopolymer (from coal fly ash) with mean adsorption energy of 1.581 kJ mol−1 and DR capacity of 17.059 mg g−1.
Interaction of hydrous titanium oxide with Cr(III) and Cr(VI) followed ion exchange mechanism with adsorption energy of 9.35 to 11.58 kJ mol−1 and 11.84 to 12.66 kJ mol−1 (288 to 318 K) respectively.255 The DR capacities varied from 1.08 to 1.24 mol kg−1 (Cr(III)) and 0.60 to 1.70 mol kg−1 (Cr(VI)) in the same temperature range.
Adsorption of Se(IV) on iron oxide (qmax: 0.109 to 0.089 mmol g−1 for 303 to 323 K) was higher compared to that on silicon oxide (qmax: 0.092 to 0.054 mmol g−1 for 303 to 323 K). Se anions interacted with the oxide surface involving adsorption of SeO32− (selenite) and HSeO3− (biselenite) on the surface by ligand-exchange. The energy of adsorption was in the range of 7.29 to 6.32 kJ mol−1 and 7.87 to 5.72 kJ mol−1 for iron oxide and silicon oxide, respectively.100
Amino-functionalized Fe3O4 magnetic nano-particles110 could take up Pb(II) with DR capacity of 105.70 mg g−1 and adsorption free energy of 10.31 kJ mol−1, with a largely chemical adsorption process.
Albadarin et al.205 reported DR capacity of dolomite for Cr(VI) of 7.35 to 3.97 mg g−1 (E = 4.87 to 4.37 kJ mol−1). Cr(VI) anions were adsorbed on >(Ca, Mg)OH2+ sites with formation of >(Ca, Mg)Cr2O7−.
Mn-diatomite had a higher DR capacity (8.06 to 9.66 mg g−1) than diatomite itself (7.30 to 8.41 mg g−1) for Zn(II) at 298 to 313 K.256 Adsorption free energies were 1.89 to 2.17 kJ mol−1 and 2.53 to 3.13 kJ mol−1 for diatomite and Mn-diatomite respectively. Both electrostatic interaction and physical adsorption were involved in the mechanism.
Mobasherpour et al.120 observed that nano hydroxyapatite could preferably adsorb Pb(II) (1003.726 mg g−1) compared to Cd(II) (150.028 mg g−1) and Ni(II) (57.812 mg g−1). The authors stated that cations with ionic radii smaller than Ca2+ (0.099 nm) showed less chance to be incorporated into the hydroxyapatite structure compared with cations with larger ionic radii. Therefore, precipitation of Ni(II) (0.072 nm) with Ca2+ would be less likely compared to that of Pb(II) (0.118 nm) and Cd(II) (0.097 nm).
DR capacities of loamy sand soil for Cr(VI) and Pb(II)124 was 1525 and 1563 mg kg−1 respectively with adsorption energy of 1400 and 335 kJ mol−1. The authors suggested formation of PbOH+ on the adsorbent surface as well as reduction of Cr(VI) to Cr(III) by natural organic matter.
DR capacity of loess soil for Cd(II) increased from 11.17 to 19.32 mg g−1 (E = 17.85 to 18.57 kJ mol−1) for temperature variation of 298 to 318 K.123 The –COOH groups of organic matter in loess soil played a significant role in the uptake of Cd(II) and the adsorption at low pH depended mainly on the protonation or deprotonation of these –COOH groups. Tang et al.202 reported DR capacity of natural Chinese loess soil for Zn(II) in the range of 0.0018 to 0.0021 mol g−1.
The presence of calcite, quartz and aragonite in the form of carbonates and oxides in beach sand created affinity for Pb(II) [qmax: (41.3 ± 21.9) to (64.1 ± 22.02) μmol g−1 for 293 to 323 K]126 and Zn(II) [qmax: 37.5 ± 9.8 μmol g−1 at 303 K].127 The mean adsorption energy was within a narrow range of 11.03 to 12.16 kJ mol−1 (Pb(II)) and 11.7 ± 0.6 kJ mol−1 (Zn(II)) supporting an ion exchange mechanism. A similar investigation of Pb(II) adsorption on river sand yielded DR capacity of 23.26 ± 0.0502 × 10−2 mmol g−1.126
Nano zerovalent iron yielded Cd(II) DR capacities of 399.10 to 473.40 mg g−1 (285 to 307 K) with E = 54.80 kJ mol−1.218 The DR capacity and adsorption energy being both large, suggested strong chemical adsorption. Fe–Ni bimetallic nanoparticles had higher Cr(VI) DR capacity (133.35 mg g−1) than those of Fe–Ni bimetallic-montmorillonite nanoparticles (129.07 mg g−1) and zero valent iron (105.70 mg g−1).62
2.4. Redlich–Peterson isotherm
When ideal monolayer adsorption is not the case, Redlich–Peterson isotherm257 is usually applied. This isotherm is considered as a combination of both Freundlich and Langmuir models and has the form,| qe = (KRP Ce)/[(1 + aRP) (Ce)g] | (9) |
(i) at g = 1, it gives the Langmuir isotherm, i.e.
| qe = (KRPCe)/[(1 + aRP)Ce] | (10) |
(ii) at g = 0, it reduces to Henry's law equation, i.e.
| qe = (KRPCe)/[1 + aRP] | (11) |
Rearranging eqn (17) to the form
| KRP(Ce/qe) − 1 = aRP(Ce)g | (12) |
Which can be converted to a linear logarithm form,258
ln[KRP(Ce/qe) − 1] = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aRP + g aRP + g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (13) |
The isotherm coefficients, aRP, KRP and g were obtained from a minimization procedure to solve this equation.
The Redlich–Peterson (RP) isotherm has not found much application because of the difficulties associated with evaluation of the three adsorption parameters. Still, some studies can be found in the literature during the last few years. In such a study, Redlich–Peterson model was applied to Cd(II) adsorption on Fe-montmorillonite and Ca-montmorillonite65 with the former having large values (Fe-montmorillonite: KRP 508.0 L mg−1, aRP 22.5 L mg−1; Ca-montmorillonite: KRP 2.37 L mg−1; aRP 0.25 L mg−1). The coefficient, g was ∼1.0 (0.959 and 0.785) suggesting adsorption mainly at specific homogeneous sites within the adsorbent. Large number and variety of –OH groups and higher CEC of Fe-montmorillonite resulted in enhanced adsorption by surface complexation and precipitation. Similarly, RP model in adsorption of Hg(II), Cd(II) and Co(II) on phosphate-immobilized-Zr-pillared bentonite yielded (i) KRP: 20.37 L mg−1, aRP: 0.517 mg−1, g: 0.94 for Hg(II), (ii) KRP: 9.98 L mg−1, aRP: 0.20 L mg−1, g: 0.99 for Cd(II) and (iii) KRP: 10.31 L mg−1, aRP: 0.226 L mg−1, g: 0.99 for Co(II). The metal uptake capacity was shown to have been boosed by phosphate immobilization creating well defined porous structure.159
Fe pillared, Fe/Cr pillared and Cr pillared bentonite were shown to adsorb Cd(II) with RP parameters, g ∼ 1.0 for all the three adsorbents (RP model reduces to Langmuir form), and KRP 1.71 L mg−1, aRP 2.28 L mmol−1 for Fe pillared bentonite, KRP 1.41 L mg−1, aRP 1.80 L mmol−1 for Fe/Cr pillared bentonite and KRP 1.34 L mg−1, aRP 3.43 L mmol−1 for Cr pillared bentonite.63 RP coefficients for adsorption of Pb(II) and Cu(II) on Urea-intercalated-delaminated kaolinite (KRP: 4.0 ± 0.2 and 3.6 ± 0.1 L mol−1), urea-intercalated kaolinite (KRP: 3.651 and 3.169 L mol−1) and natural kaolinite (KRP: 2.0 ± 0.3 and 2.2 ± 0.2 L mol−1) showed appreciable differences.154
Dodecylamine modified sodium montmorillonite was shown to adsorb Cr(VI) with KRP, aRP and g of 2.596 L g−1, 0.184 L mg−1 and 0.86 respectively.73 Cr(VI) anions were held electrostatically to the protonated dodecylamine and surface hydroxyl groups in montmorillonite.
The RP parameters for adsorption of Cr(VI) and Cr(III) on hydrous titanium oxide at 288 K were significantly different, KRP: 2.419 L mg−1, aRP: 0.222 L mg−1, g: 1.082 than for Cr(VI) and KRP: 0.764 L mg−1, aRP: 0.036 L mg−1, g: 1.004 for Cr(III).255 Ni(II) adsorbed on the same material at 288 ± 1 to 328 ± 1 with RP parameters of 34.83 to 47.24 L g−1 (KRP), 4.32 to 2.30 L mg−1 (aRP) and 0.82 to 0.93 (g).259
Pb(II) adsorption on natural apatite and synthetic hydroxyapatite had KRP: 0.532 L mg−1, aRP: 3.05 L mg−1, g: 0.410 and KRP: 38.7 L mg−1, aRP: 82.6 L mg−1, g: 0.797 respectively.121
Guerra et al.179 reported Pb(II), Mn(II) and Zn(II) adsorption on 3-aminopropyltriethoxysilane modified nontronite with KRP (L g−1) of Pb 25.034, Mn 23.916, Zn 22.218; aRP ({mmol L−3}−g) of Pb 0.984, Mn 0.988, Zn 0.993 while the parent nontronite had KRP (L g−1): Pb 13.451, Mn 13.925, Zn 12.217; aRP ({mmol L−3}−g): Pb 0.812, Mn 0.889, Zn 0.951. The g values were ∼1.0. Organic functionalization was seen to have positive influence on KRP.
Amino functionalized silica gel with three different loadings of aminopropyl group yielded KRP from 0.062 to 0.002 L g−1 (g = 0.95) for Cu(II).115 Another material, aminated mesoporous alumina adsorbed Cu(II) with KRP 2.22 L mg−1, aRP 0.55 L mg−1, g 0.80 while Cu(II) on simple protonated mesoporous alumina had KRP 2.63 L mg−1, aRP 0.37 L mg−1, g 0.90.112 Mg-mesoporous alumina held Ni(II) with KRP: 9.93 L g−1, aRP: 2.18 L g−1.260 The large values were explained on the basis of interconnected pore system of mesoporous alumina that reduced transport limitations and enhanced the accessibility of the active sites.
Cr(VI) adsorption on zerovalent iron, Fe–Ni bimetallic nanoparticles and Fe–Ni bimetallic-montmorillonite nanoparticles KRP of 3.16 × 103 to 4.60 × 103 L g−1; aRP: 56.88 to 64.71 L mg−1 with g ∼ 1.0.62
2.5. Temkin isotherm
Temkin isotherm261 is based on the assumption that the adsorption heat decreases linearly with coverage instead of a logarithmic decrease as implied in the Freundlich isotherm262,263 and that there is a uniform distribution of binding energies. The isotherm has the form,264| qe = (RT/bT)ln(KTCe) | (14) |
qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KT + B KT + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (15) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce can be used to determine the values of KT and B. Temkin isotherm requires that the adsorption process is accompanied by a uniform distribution of binding energies, which is hardly possible while considering adsorption from solutions on to a solid. Thus, while Temkin equation is excellent for predicting the gas phase equilibrium (when organization in a tightly packed structure with identical orientation is not necessary), it is found that complex systems, particularly liquid-phase adsorption equilibria are usually not appropriate to be represented by Temkin isotherm.265
Ce can be used to determine the values of KT and B. Temkin isotherm requires that the adsorption process is accompanied by a uniform distribution of binding energies, which is hardly possible while considering adsorption from solutions on to a solid. Thus, while Temkin equation is excellent for predicting the gas phase equilibrium (when organization in a tightly packed structure with identical orientation is not necessary), it is found that complex systems, particularly liquid-phase adsorption equilibria are usually not appropriate to be represented by Temkin isotherm.265
It is thus found in the literature that in those few cases (of adsorption from solutions) where application of Temkin isotherm was attempted, the interpretations of the results were inconsistent and incomplete, particularly with respect to values of the Temkin coefficieny, KT.62,73,89,99,121,158,169,218,254,260
2.6. Toth isotherm
To obtain a general relationship for the interactions of adsorptive molecules and the energetically heterogeneous solid surfaces, Toth266 observed that a heterogeneous surface would have a higher uptake of adsorptive at the same relative equilibrium pressure than a homogeneous surface. The Toth isotherm is strictly applicable to adsorption of gases on solids, but the equation has been extended to adsorption from solutions with the following common form:255| qe = (QmaxbT, Ce)/([1 + (bTCe)1/n]n) | (16) |
| Ce1/n/qe = [1/(QmaxbT)] + (1/Qmax)Ce | (17) |
The three-parameter Toth equation is not easy to solve and this has resulted in only a few applications of the same. Debnath and Ghosh255 reported Toth capacities of hydrous TiO2 for Cr(III) and Cr(VI) in the range of 21.71 mg g−1 to 64.13 mg g−1 and 10.43 mg g−1 to 29.99 mg g−1 respectively (288 K to 318 K). The Toth coefficient, n, had values of Cr(III): 1.185 to 1.474 and Cr(VI): 0.987 to 1.029.
Montmorillonite–Cu(II) interaction yielded Toth capacity of 25.124 mg g−1 with n = 1.230.252 Cu(II) cations were adsorbed by an ion exchange mechanism at permanent charge sites and also by the formation of complexes with surface hydroxyl groups of the adsorbent. The Toth capacity of zeolite for Pb(II) (0.433 mmol g−1) was found to be higher than that for Cu(II) (0.410 mmol g−1) and Zn(II) (0.201 mmol g−1) with ‘n’ varying between 0.90 to 0.99.253
3. Molecular simulation of adsorption
There are some studies in which molecular simulation has been used to predict the adsorption isotherms. However, the output of a simulation is the absolute number of molecules present in the framework, whereas experimental results are often reported as the excess amount adsorbed.267Kremleva et al.268 carried out DF calculations on supercell slab models of kaolinite edge surfaces, using the plane-wave based ab initio simulation to interactions of uranyl groups with kaolinite edge surfaces. The (010) surface of kaolinite edge facets was expected to be highly reactive that could preferentially adsorb metal cations. In considering bidentate uranyl adsorption complexes at (010) edge surfaces, three types of deprotonated adsorption sites, namely, (i) pure aluminol (Al–OH) sites, (ii) mixed silanol–aluminol sites within the same substrate layer of kaolinite and (iii) bridging silanol–aluminol sites between neighboring layers, were taken into consideration. Adsorption at the bridging sites was found to be essentially as stable as complexes at aluminol sites, while complexes at mixed aluminol–silanol sites within a single kaolinite layer were energetically less favorable. Uranyl adsorption was accompanied by hydrolysis resulting in uranyl mono- or dihydroxide as the adsorbate. Comparison of energetically preferred adsorption complexes on the (001) and (010) surfaces of kaolinite showed no characteristic structural feature that would differentiate between uranyl adsorption on these two types of surfaces.
The ab initio molecular dynamics simulation for Li+-, Na+- and K+-montmorillonites was studied by considering three types of isomorphic substitutions in the montmorillonite layer: tetrahedral (Tsub), octahedral (Osub) and both (OTsub).269 The authors observed that for all types of montmorillonites, K+ remained bound to the surface, confirming its role as a swelling inhibitor. On the other hand, Li+ showed a tendency to hydrate and coordinate to four H2O molecules in all the cases, except for the OTsub substituted montmorillonite where one of the two Li+ cations remained bound to the oxygen atoms close to the substituted tetrahedral site. Na+ possessed an intermediate behaviour and could bind to the surface for Tsub montmorillonite but got hydrated for Osub. The inner/outer complex formation of the cations depended on the relative water-cation and surface-cation affinity. The binding energy between alkali cations and water was highest for Li+, followed by Na+ and K+, the ions being bound to 4, 5, and 6 solvating water molecules, respectively. Moreover, increase in the cation size and polarizability, enhances the interactions with the surface, following the sequence Li+ < Na+ < K+. From the molecular electrostatic potential maps, it was shown that the effects of tetrahedral and/or octahedral substitutions in montmorillonite were to adjust the negative charge carried by the surface oxygen atoms, thereby tuning the cation-surface affinity and the inner/outer complex formation.
The adsorption behaviour of V(V) on goethite surface and the acid–base behaviour of the adsorbent surface were theoretically studied by Peacock and Sherman.270 It was shown that at pH ∼ 6.0 to 9.0, adsorption was concentration dependent that reflected the formation of polynuclear complexes in solution. V(V) adsorbed on α-FeOOH surface as the mononuclear VO2+ (pH 1.0 to 4.0) and VO3(OH)2− ions (pH > 4.0). Adsorption was accompanied by the formation of inner-sphere surface complexes resulting from bidentate corner-sharing between doubly and singly protonated VO43− tertrahedra and FeO6 polyhedra. The authors fitted the experimental V(V) adsorption data to the reactions,
| 2FeOH2+ + VO2 = Fe2O2V(OH)2+ + 2H+ |
| 2FeOH + HVO42− = Fe2O2VO(OH)0 + 2OH− |
The same group of authors investigated adsorption of Cu(II) onto goethite (α-FeOOH), hematite (α-Fe2O3) and lepidocrocite (γ-FeOOH) at pH 2.0–7.0 and showed that Cu(II) adsorbed as (CuO4Hn)n−6 and binuclear (Cu2O6Hn)n−8 complexes.271 These could form inner-sphere complexes with iron (hydr)oxide surfaces by corner-sharing with two or three edge-sharing Fe(O,OH)6 polyhedra. No evidences was found for surface complexes resulting from either monodentate corner sharing or bidentate edge-sharing between (CuO4Hn)n−6 and Fe(O,OH)6 polyhedra. With the bidentate (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeOH)2Cu(OH)20 and tridentate (
FeOH)2Cu(OH)20 and tridentate (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3O(OH)2)Cu2(OH)30 surface complexes, the experimental Cu(II) adsorption data could be expressed as,
Fe3O(OH)2)Cu2(OH)30 surface complexes, the experimental Cu(II) adsorption data could be expressed as,
3(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeOH) + 2Cu2+ + 3H2O → ( FeOH) + 2Cu2+ + 3H2O → (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3O(OH)2)Cu2(OH)30 + 4H+ Fe3O(OH)2)Cu2(OH)30 + 4H+ |
2(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeOH) + 2Cu2+ + 2H2O → ( FeOH) + 2Cu2+ + 2H2O → (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3OH2)Cu(OH)20 + 2H+ Fe3OH2)Cu(OH)20 + 2H+ |
The non-coulombic interactions of Cd(II), Cu(II) and Zn(II) with (001) surface of muscovite mica were studied by atomistic simulation techniques and a set of specific interatomic potentials.272 The surface experienced only minimal relaxation. The study indicated absence of strong bonding of Cd(II), Cu(II) and Zn(II) to the perfect (001) surface of muscovite. The bond lengths (2.6 and 2.8 Å) were much longer than those observed by experiment suggesting that only weak interactions exist between metal cation and adsorbent surface, pointing to physisorption rather than chemisorption. The lack of reactivity was explained by considering the stability of the SiO2 units that make up the surface in which all oxygen bonds were satisfied. The replacements of 25% Si by Al imparted some residual charge to the surface, which was not enough to lead to chemisorption. Adsorption onto 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layered silicate surface was enhanced by isomorphic charge distribution and higher pH as collaborated by experimental results. The increased adsorption at pH > 6.0 indicated complexation of metal cations to AlOH and SiOH groups at edge sites of mica. Stable defects on a surface could offer new sites for the binding of metal cations and Cu(II) and Zn(II) might be attached to these sites forming four M–O bonds. On the basis of the solvated structures, the authors concluded that the binding of Cu(II) and Zn(II) from solution and the associated loss of K+ ions into solution were favoured. Zn(II) could combine more strongly than Cu(II). The theoretical study also indicated that Cd(II) did not chemisorb into the defect sites, which might be due to the separation between the oxygens of the tetrahedral layer and those of the octahedral layer, which selectively accommodate smaller ions. Cd(II) could form a much less stable structure on the mica surface by binding to two oxygen atoms of the defect sites and the stability of this configuration was increased by the presence of water molecules. Cd(II) preferably formed outer sphere complexes rather to bind directly with either the smooth muscovite surface or edge sites, which was supported by experimental data.
1 layered silicate surface was enhanced by isomorphic charge distribution and higher pH as collaborated by experimental results. The increased adsorption at pH > 6.0 indicated complexation of metal cations to AlOH and SiOH groups at edge sites of mica. Stable defects on a surface could offer new sites for the binding of metal cations and Cu(II) and Zn(II) might be attached to these sites forming four M–O bonds. On the basis of the solvated structures, the authors concluded that the binding of Cu(II) and Zn(II) from solution and the associated loss of K+ ions into solution were favoured. Zn(II) could combine more strongly than Cu(II). The theoretical study also indicated that Cd(II) did not chemisorb into the defect sites, which might be due to the separation between the oxygens of the tetrahedral layer and those of the octahedral layer, which selectively accommodate smaller ions. Cd(II) could form a much less stable structure on the mica surface by binding to two oxygen atoms of the defect sites and the stability of this configuration was increased by the presence of water molecules. Cd(II) preferably formed outer sphere complexes rather to bind directly with either the smooth muscovite surface or edge sites, which was supported by experimental data.
Kaludjerovic-Radoicic and Raicevic121 studied the adsorption of Pb(II) on natural apatite and hydroxyapatite and the experiments were simulated in which 0.2 g of adsorbent reacted with 0.483 mmol Pb(II) L−1 (as nitrate). According to the Visual MINTEQ calculations, 99.97% of Pb(II) could be adsorbed on hydroxyapatite, while the experimental value was 99.60%. The calculations showed the presence of two solids at equilibrium, namely, pyromorphite (HPM) and hydroxyapatite (HAP), and that HAP dissolved to form HPM until there were Pb(II) present in the solution. Only 0.03% of initial Pb(II) concentration remained in solution, indicating that the main mechanism of Pb(II) removal from the solution was dissolution of HAP followed by HPM precipitation, though the experimental data did not show any new solid phase formation. The reason for this could be the low concentration of HPM or the poor crystallinity of the newly formed solid phase. Visual MINTEQ calculations showed that synthetic HAP was an appropriate adsorbent for Pb(II). However, a significant difference between calculated and experimental values of Pb(II) removal was observed for natural apatite (calculated: 99.95%, experimental: 41.6%). Visual MINTEQ predicted two solids at Pb(II)–apatite equilibrium, namely, fluorapatite and pyromorphite. According to the calculations, natural apatite dissolved to provide PO43− ions for HPM formation. The large difference between experimental and calculated values of Pb(II) removal indicated that dissolution–precipitation mechanism suggested by this model could not describe Pb(II)–natural apatite interactions. Adsorption of Pb(II) by HAP was shown to lead to encapsulation of HAP particles by insoluble pyromorphite, preventing the process of Pb(II) immobilization. On the other hand, apatite could form apatite/Pb(II) solid solution instead of the pyromorphite, which allow diffusion of Pb(II) from the surface into the HAP particles resulting in slow but continuous uptake of Pb(II).
Dou et al.273 reported that As(V) primarily reacted with Fe–OH sites of Fe–Ce bimetal oxide with the formation of monodentate mononuclear and bidentate binuclear surface complexes of As(V), which coexisted. The existence of monodentate complex might be due to the existence of Ce atoms in the bimetal oxide. Moreover, molecular simulation studies showed that protonated monodentate complex and deprotonated bidentate complex preferred to co-exist at Fe–Ce surface under a significantly high surface loading. Protonated monodentate complex was found to be dominant at pH < 8.0, while deprotonated bidentate complex was dominant at pH > 8.0. By increasing the protonated monodentate complex, As(V) surface loading could be increased.
4. Conclusion and future perspective
From the large number of works reviewed here, it is observed that the adsorption capacities of metal cations and anions on inorganic adsorbents are mainly evaluated on the basis of Freundlich and Langmuir isotherm models. The maximum uptake capacities of different adsorbents provide some idea of their effectiveness for each type of metal cation/anion. Most of these adsorbents have good removal capacities, sometimes even better than activated carbons. Different modification processes often enhance the uptake capacities; however, the reverse observations are also there.Although the suitability of the equilibrium isotherm model is important for a specific adsorbate–adsorbent process, some adsorption studies have been reported without the use of any isotherm model. These have not been covered in this review.
Despite the fact that the influences of experimental parameters on adsorption capacities for various adsorbate–adsorbent systems are not very straight forward, yet some general conclusions can be drawn. A few of them are;
(i) The nature of the surface functional groups considerably influences adsorption of cations and anions.
(ii) Pre-treatment (e.g. acid-treatment, pillaring, organic functionalization, etc.) of the solid in various ways serve to change the number and distribution of surface active sites and thereby, influences adsorption capacities.
(iii) The solution pH affects the adsorbent surface by placing a layer of hydroxyl or hydrogen ions on the surface which influences adsorption of cations and anions as well as the mechanism of uptake.
(iv) Increase in solution temperature enhances the mobility of the ions and transport limitation, if any, is minimized. Results indicate both increase and decrease in the adsorption capacities of solids with change in temperature.
(v) Adsorption capacity is likely to be affected by the way in which the adsorbate–adsorbent mixture was agitated (e.g. stirring speed). However, this angle was not studied much except in one or two studies.
This literature review shows remarkable variations in the mechanisms of adsorption from one system to another. The mechanisms suggested belong to ion exchange, ligand exchange, electrostatic interaction, formation of inner and outer sphere complexes, chelation, hydrogen bonding, acid–base interaction, etc., and in many cases, more than one mechanism has been predicted as operating simultaneously. There are large differences in how the adsorption capacities are represented and in a considerable number of publications, no attention seems to have been given to use a uniform pattern of units, many have just ignored the use of units.
Nomenclature
| Ce | Equilibrium liquid phase concentration |
| qe | Solid phase concentration |
| KF | Freundlich adsorption capacity |
| n | Freundlich adsorption intensity, Toth coefficient |
| qm | Langmuir capacity |
| b | Langmuir adsorption equilibrium constant |
| qmax | Dubinin–Radushkevich capacity |
| β | Dubinin–Radushkevich coefficient related to the mean free energy of adsorption per mol of the adsorbate |
| ε | Polanyi potential |
| R | Universal gas constant |
| K | Absolute temperature |
| E | Mean free energy |
| KRP | Redlich–Peterson coefficient |
| aRP | Redlich–Peterson coefficient |
| g | Redlich–Peterson heterogeneity index |
| KT | Temkin equilibrium binding energy |
| B | Temkin coefficient related to enthalpy of adsorption |
| Qmax | Toth capacity |
| bT | Toth coefficient |
| CEC | Cation exchange capacity |
References
- S. Hong, C. Wen, H. Jing, F. Gan and Y.-S. Ho, J. Hazard. Mater., 2009, 167, 630–633 CrossRef CAS PubMed.
- M. I. El-Khaiary, J. Hazard. Mater., 2008, 158, 73–87 CrossRef CAS PubMed.
- K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2010, 156, 2–10 CrossRef CAS PubMed.
- W. J. Weber, Jr, Adsorption theory, concepts, and models, in Adsorption Technology: A Step-by-Step Approach to Process Evaluation and Application, ed. F. L. Slejko, Marcel Dekker, New York, 1985, p. 1 Search PubMed.
- F. E. Bernardin, Jr, Experimental design and testing of adsorption and adsorbates, in Adsorption Technology: A Step-by-Step Approach to Process Evaluation and Application, ed. F. L. Slejko, Marcel Dekker, New York, 1985, p. 37 Search PubMed.
- W. J. Thomas and B. Crittenden, Fundamental of adsorption equilibria, in Adsorption Technology and Design, Butterworth-Heinemann, Oxford, 1998, ch. 3, p. 31 Search PubMed.
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS PubMed.
- Q. U. Jiuhui, J. Environ. Sci., 2008, 20, 1–13 CrossRef CAS.
- V. K. Gupta, P. J. M. Carrott, M. M. L. R. Carrott and Suhas, Crit. Rev. Environ. Sci. Technol., 2009, 39, 783–842 CrossRef.
- A. Bhatnagar and M. Sillanpää, Chem. Eng. J., 2010, 157, 277–296 CrossRef CAS PubMed.
- S. Wang and Y. Peng, Chem. Eng. J., 2010, 156, 11–24 CrossRef CAS PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Adv. Colloid Interface Sci., 2008, 140, 114–131 CrossRef CAS PubMed.
- S. Sen Gupta and K. G. Bhattacharyya, Phys. Chem. Chem. Phys., 2012, 14, 6698–6723 RSC.
- S. Sen Gupta and K. G. Bhattacharyya, Natural and treated montmorillonites as scavengers of toxic metals from water, in Application of Adsorbents for Water Pollution Control, ed. A. Bhatnagar, Bentham Science Publishers, USA, 2012, p. 291 Search PubMed.
- M. Borowko, Adsorption on heterogeneous surfaces, in Adsorption Theory, Modelling, and Analysis, Surfactant Science Series, ed. J. Toth, Marcel Dekker, New York, 2002, vol. 107, p. 105 Search PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121–129 CrossRef CAS.
- J. H. De Boer, The Dynamical Character of Adsorption, Oxford University Press, London, 2nd edn, 1968 Search PubMed.
- M. M. Dubinin, Chem. Rev., 1960, 60, 235–266 CrossRef CAS.
- D. M. Ruthven, Principles of Adsorption and Adsorption Processes, Wiley, New York, 1984 Search PubMed.
- H. M. F. Freundlich, J. Phys. Chem., 1906, 57, 385–470 CAS.
- W. J. Weber, Jr, P. M. McGinley and L. E. Katz, Water Res., 1991, 25, 499–528 CrossRef.
- A. W. Adamson and A. P. Gast, Physical Chemistry of Surfaces, Wiley-Interscience, New York, 1997 Search PubMed.
- J. Zeldowitsch, Acta Physicochim. URSS, 1934, 1, 961–973 Search PubMed.
- Md. Ahmaruzzaman, Adv. Colloid Interface Sci., 2008, 143, 48–67 CrossRef CAS PubMed.
- H. Jobstmann and B. Singh, Water, Air, Soil Pollut., 2001, 131, 203–215 CrossRef CAS.
- V. Chantawong, N. W. Harvey and V. N. Bashkin, Water, Air, Soil Pollut., 2003, 148, 111–125 CrossRef CAS.
- P. Turan, M. Doğan and M. Alkan, J. Hazard. Mater., 2007, 148, 56–63 CrossRef CAS PubMed.
- F. Arias and T. K. Sen, Colloids Surf., A, 2009, 348, 100–108 CrossRef CAS PubMed.
- A. Sari, M. Tuzen, D. Citak and M. Soylak, J. Hazard. Mater., 2007, 149, 283–291 CrossRef CAS PubMed.
- A. Sarı, M. Tuzen and M. Soylak, J. Hazard. Mater., 2007, 144, 41–46 CrossRef PubMed.
- S. Wang, Y. Dong, M. He, L. Chen and X. Yu, Appl. Clay Sci., 2009, 43, 164–171 CrossRef CAS PubMed.
- S. M. Dal Bosco, R. S. Jimenez, C. Vignado, J. Fontana, B. Geraldo, F. C. A. Figueiredo, D. Mandelli and W. A. Carvalho, Adsorption, 2006, 12, 133–146 CrossRef CAS.
- Ş. Kubilay, R. Gürkan, A. Savran and T. Şahan, Adsorption, 2007, 13, 41–51 CrossRef.
- T. K. Sen and D. Gomez, Desalination, 2011, 267, 286–294 CrossRef CAS PubMed.
- N. Karapinar and R. Donat, Desalination, 2009, 249, 123–129 CrossRef CAS PubMed.
- J. Li, J. Hu, G. Sheng, G. Zhao and Q. Huang, Colloids Surf., A, 2009, 349, 195–201 CrossRef CAS PubMed.
- S. Yang, D. Zhao, H. Zhang, S. Lu, L. Chen and X. Yu, J. Hazard. Mater., 2010, 183, 632–640 CrossRef CAS PubMed.
- S. Veli and B. Alyüz, J. Hazard. Mater., 2007, 149, 226–233 CrossRef CAS PubMed.
- S. Q. Zhang and W. G. Hou, Colloids Surf., A, 2008, 320, 92–97 CrossRef CAS PubMed.
- S. I. Abu-Eishah, Appl. Clay Sci., 2008, 42, 201–205 CrossRef CAS PubMed.
- Q. Fan, D. Shao, Y. Lu, W. Wu and X. Wang, Chem. Eng. J., 2009, 150, 188–195 CrossRef CAS PubMed.
- J.-S. Yang, J. Y. Lee, Y.-T. Park, K. Baek and J. Choi, Sep. Sci. Technol., 2010, 45, 814–823 CrossRef CAS.
- M.-q. Jiang, Q.-p. Wang, X.-y. Jin and Z.-l. Chen, J. Hazard. Mater., 2009, 170, 332–339 CrossRef CAS PubMed.
- R. A. Shawabkeh, O. A. Al-Khashman, H. S. Al-Omari and A. F. Shawabkeh, Environmentalist, 2007, 27, 357–363 CrossRef PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Ind. Eng. Chem. Res., 2007, 46, 3734–3742 CrossRef CAS.
- S. Sen Gupta and K. G. Bhattacharyya, J. Hazard. Mater., 2006, 128, 247–257 CrossRef CAS PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Appl. Clay Sci., 2008, 41, 1–9 CrossRef CAS PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Colloids Surf., A, 2008, 317, 71–79 CrossRef CAS PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Sep. Sci. Technol., 2007, 42, 3391–3418 CrossRef CAS.
- K. G. Bhattacharyya and S. Sen Gupta, Sep. Sci. Technol., 2008, 43, 3221–3250 CrossRef CAS.
- K. G. Bhattacharyya and S. Sen Gupta, Adsorption, 2006, 12, 185–204 CrossRef CAS.
- S. Sen Gupta and K. G. Bhattacharyya, Appl. Clay Sci., 2005, 30, 199–208 CrossRef CAS PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Colloids Surf., A, 2006, 277, 191–200 CrossRef CAS PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Ind. Eng. Chem. Res., 2006, 45, 7232–7240 CrossRef CAS.
- K. G. Bhattacharyya and S. Sen Gupta, Sep. Purif. Technol., 2006, 50, 388–397 CrossRef CAS PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Desalination, 2011, 272, 66–75 CrossRef CAS PubMed.
- B. I. Olu-Owolabi, D. B. Popoola and E. I. Unuabonah, Water, Air, Soil Pollut., 2010, 211, 459–474 CrossRef CAS.
- E. Eren, A. Tabak and B. Eren, Desalination, 2010, 257, 163–169 CrossRef CAS PubMed.
- M. Ranđelović, M. Purenović, A. Zarubica, J. Purenović, B. Matović and M. Momćilović, J. Hazard. Mater., 2012, 199–200, 367–374 CrossRef PubMed.
- A. Mockovčiaková, Z. Orolínová and J. Škvarla, J. Hazard. Mater., 2010, 180, 274–281 CrossRef PubMed.
- B. S. Kadu and R. C. Chikate, J. Environ. Chem. Eng., 2013, 1, 320–327 CrossRef CAS PubMed.
- F. Tomul, Ind. Eng. Chem. Res., 2011, 50, 7228–7240 CrossRef CAS.
- L.-g. Yan, X.-q. Shan, B. Wen and G. Owens, J. Hazard. Mater., 2008, 156, 499–508 CrossRef CAS PubMed.
- P. Wu, W. Wu, S. Li, N. Xing, N. Zhu, P. Li, J. Wu, C. Yang and Z. Dang, J. Hazard. Mater., 2009, 169, 824–830 CrossRef CAS PubMed.
- P. Wu, Q. Zhang, Y. Dai, N. Zhu, Z. Dang, P. Li, J. Wu and X. Wang, Geoderma, 2011, 164, 215–219 CrossRef CAS PubMed.
- B. Hu, H. Luo, H. Chen and T. Dong, Appl. Clay Sci., 2011, 51, 198–201 CrossRef CAS PubMed.
- J. Su, H.-G. Huang, X.-Y. Jin, X.-Q. Lu and Z.-L. Chen, J. Hazard. Mater., 2011, 185, 63–70 CrossRef CAS PubMed.
- S. Sen Gupta and K. G. Bhattacharyya, J. Colloid Interface Sci., 2006, 295, 21–32 CrossRef CAS PubMed.
- Ö. Gök, A. Özcan, B. Erdem and A. S. Özcan, Colloids Surf., A, 2008, 317, 174–185 CrossRef PubMed.
- A. S. Özcan, Ö. Gök and A. Özcan, J. Hazard. Mater., 2009, 161, 499–509 CrossRef PubMed.
- B. Erdem, A. Özcan, Ö. Gök and A. S. Özcan, J. Hazard. Mater., 2009, 163, 418–426 CrossRef CAS PubMed.
- A. S. K. Kumar, R. Ramachandran, S. Kalidhasan, V. Rajesh and N. Rajesh, Chem. Eng. J., 2012, 211–212, 396–405 CrossRef PubMed.
- W. A. Carvalho, C. Vignado and J. Fontana, J. Hazard. Mater., 2008, 153, 1240–1247 CrossRef CAS PubMed.
- M. Şölener, S. Tunali, A. S. Özcan, A. Özcan and T. Gedikbey, Desalination, 2008, 223, 308–322 CrossRef PubMed.
- W. Li, Y. Tang, Y. Zeng, Z. Tong, D. Liang and W. Cui, Chem. Eng. J., 2012, 193–194, 88–95 CAS.
- V. Marjanović, S. Lazarević, I. Janković-Častvan, B. Potkonjak, Đ. Janaćković and R. Petrović, Chem. Eng. J., 2011, 166, 198–206 CrossRef PubMed.
- V. Marjanović, S. Lazarević, I. Janković-Častvan, B. Jokić, D. Janaćković and R. Petrović, Appl. Clay Sci., 2013, 80–81, 202–210 CrossRef PubMed.
- X. Liang, Y. Xu, L. Wang, Y. Sun, D. Lin, Y. Sun, X. Qin and Q. Wan, Chemosphere, 2013, 90, 548–555 CrossRef CAS PubMed.
- M. Liu, L. Hou, B. Xi, Y. Zhao and X. Xia, Appl. Surf. Sci., 2013, 273, 706–716 CrossRef CAS PubMed.
- M. A. Shavandi, Z. Haddadian, M. H. S. Ismail, N. Abdullah and Z. Z. J. Abidin, J. Taiwan Inst. Chem. Eng., 2012, 43, 750–759 CrossRef CAS PubMed.
- M. Karatas, J. Hazard. Mater., 2012, 199–200, 383–389 CrossRef CAS PubMed.
- S. Wang, T. Terdkiatburana and M. O. Tadè, Sep. Purif. Technol., 2008, 62, 64–70 CrossRef CAS PubMed.
- D. Nibou, H. Mekatel, S. Amokrane, M. Barkat and M. Trari, J. Hazard. Mater., 2010, 173, 637–646 CrossRef CAS PubMed.
- M. E. Argun, J. Hazard. Mater., 2008, 150, 587–595 CrossRef CAS PubMed.
- Y.-h. Xu, T. Nakajima and A. Ohki, J. Hazard. Mater., 2002, 92, 275–287 CrossRef CAS.
- A. Nezamzadeh-Ejhieh and M. Kabiri-Samani, J. Hazard. Mater., 2013, 260, 339–349 CrossRef CAS PubMed.
- J. Lin, Y. Zhan and Z. Zhu, Colloids Surf., A, 2011, 384, 9–16 CrossRef CAS PubMed.
- H. Javadian, F. Ghorbani, H.-a. Tayebi and S. M. H. Asl, Arabian J. Chem., 2013 DOI:10.1016/j.arabjc.2013.02.018 , article in press.
- M. R. Panuccio, A. Sorgonà, M. Rizzo and G. Cacco, J. Environ. Manage., 2009, 90, 364–374 CrossRef CAS PubMed.
- L. A. Rodrigues, L. J. Maschio, R. E. da Silva and M. L. C. P. da Silva, J. Hazard. Mater., 2010, 173, 630–636 CrossRef CAS PubMed.
- T. Sheela, Y. A. Nayaka, R. Viswanatha, S. Basavanna and T. G. Venkatesha, Powder Technol., 2012, 217, 163–170 CrossRef CAS PubMed.
- V. Venkatesham, G. M. Madhu, S. V. Satyanarayana and H. S. Preetham, Procedia Eng., 2013, 51, 308–313 CrossRef CAS PubMed.
- T. Sheela and Y. A. Nayaka, Chem. Eng. J., 2012, 191, 123–131 CrossRef CAS PubMed.
- T. K. Sen and M. V. Sarzali, Chem. Eng. J., 2008, 142, 256–262 CrossRef CAS PubMed.
- Q. Su, B. Pan, S. Wan, W. Zhang and L. Lv, J. Colloid Interface Sci., 2010, 349, 607–612 CrossRef CAS PubMed.
- Q. Chen, D. Yinb, S. Zhu and X. Hu, J. Colloid Interface Sci., 2012, 367, 241–248 CrossRef CAS PubMed.
- A. Nilchi, S. R. Garmarodi and S. J. Darzi, Sep. Sci. Technol., 2010, 45, 801–808 CrossRef CAS.
- W. Liu, T. Wang, A. G. L. Borthwick, Y. Wang, X. Yin, X. Li and J. Ni, Sci. Total Environ., 2013, 456–457, 171–180 CrossRef CAS PubMed.
- R. R. Sheha and E. A. El-Shazly, Chem. Eng. J., 2010, 160, 63–71 CrossRef CAS PubMed.
- N. N. Nassar, J. Hazard. Mater., 2010, 184, 538–546 CrossRef CAS PubMed.
- I. Akin, G. Arslan, A. Tor, M. Ersoz and Y. Cengeloglu, J. Hazard. Mater., 2012, 235–236, 62–68 CrossRef CAS PubMed.
- Y.-H. Huang, C.-L. Hsueh, C.-P. Huang, L.-C. Su and C.-Y. Chen, Sep. Purif. Technol., 2007, 55, 23–29 CrossRef CAS PubMed.
- S. Zhang, H. Niu, Y. Cai, X. Zhao and Y. Shi, Chem. Eng. J., 2010, 158, 599–607 CrossRef CAS PubMed.
- Y. Zhang, M. Yang and X. Huang, Chemosphere, 2003, 51, 945–952 CrossRef CAS.
- Q. Zhang, B. Pan, W. Zhang, B. Pan, Q. Zhang and H. Ren, Ind. Eng. Chem. Res., 2008, 47, 3957–3962 CrossRef CAS.
- M. Ozmen, K. Can, G. Arslan, A. Tor, Y. Cengeloglu and M. Ersoz, Desalination, 2010, 254, 162–169 CrossRef CAS PubMed.
- Y. Jin, F. Liu, M. Tong and Y. Hou, J. Hazard. Mater., 2012, 227–228, 461–468 CrossRef CAS PubMed.
- A. Z. M. Badruddoza, Z. B. Z. Shawon, T. W. J. Daniela, K. Hidajat and M. Shahab Uddin, Carbohydr. Polym., 2013, 91, 322–332 CrossRef CAS PubMed.
- Y. Tan, M. Chen and Y. Hao, Chem. Eng. J., 2012, 191, 104–111 CrossRef CAS PubMed.
- T. K. Naiya, A. K. Bhattacharya and S. K. Das, J. Colloid Interface Sci., 2009, 333, 14–26 CrossRef CAS PubMed.
- S. Rengaraj, Y. Kim, C. K. Joo and J. Yi, J. Colloid Interface Sci., 2004, 273, 14–21 CrossRef CAS PubMed.
- A. Jaiswal, S. Banerjee, R. Mani and M. C. Chattopadhyaya, J. Environ. Chem. Eng., 2013, 1, 281–289 CrossRef CAS PubMed.
- F. Granados-Correa, N. G. Corral-Capulin, M. T. Olguín and C. E. Acosta-León, Chem. Eng. J., 2011, 171, 1027–1034 CrossRef CAS PubMed.
- V. Manu, H. M. Mody, H. C. Bajaj and R. V. Jasra, Ind. Eng. Chem. Res., 2009, 48, 8954–8960 CrossRef CAS.
- A. Heidari, H. Younesi and Z. Mehraban, Chem. Eng. J., 2009, 153, 70–79 CrossRef CAS PubMed.
- X. Liang, Y. Xu, G. Sun, L. Wang, Y. Sun and X. Qin, Colloids Surf., A, 2009, 349, 61–68 CrossRef CAS PubMed.
- T. S. Anirudhan and P. S. Suchithra, Indian J. Chem. Technol., 2010, 17, 247–259 CAS.
- D. Zhao, G. Sheng, J. Hu, C. Chen and X. Wang, Chem. Eng. J., 2011, 171, 167–174 CrossRef CAS PubMed.
- I. Mobasherpour, E. Salahi and M. Pazouki, Arabian J. Chem., 2012, 5, 439–446 CrossRef CAS PubMed.
- T. Kaludjerovic-Radoicic and S. Raicevic, Chem. Eng. J., 2010, 160, 503–510 CrossRef CAS PubMed.
- C. P. Wang, J. Z. Wu, H. W. Sun, T. Wang, H. B. Liu and Y. Chang, Ind. Eng. Chem. Res., 2011, 50, 8515–8523 CrossRef CAS.
- Y. Wang, X. Tang, Y. Chen, L. Zhan, Z. Li and Q. Tang, J. Hazard. Mater., 2009, 172, 30–37 CrossRef CAS PubMed.
- B. Fonseca, H. Maio, C. Quintelas, A. Teixeira and T. Tavares, Chem. Eng. J., 2009, 152, 212–219 CrossRef CAS PubMed.
- R. Ahmed, T. Yamin, M. S. Ansari and S. M. Hasany, Adsorpt. Sci. Technol., 2006, 24, 475–486 CrossRef CAS.
- S. I. H. Taqvi, M. I. Bhanger, S. W. Shah and S. M. Hasany, Sep. Sci. Technol., 2006, 41, 531–547 CrossRef CAS.
- S. I. H. Taqvi, S. M. Hasany and M. I. Bhanger, Sep. Purif. Technol., 2008, 61, 153–160 CrossRef CAS PubMed.
- S.-M. Lee, C. Laldawngliana and D. Tiwari, Chem. Eng. J., 2012, 195–196, 103–111 CrossRef CAS PubMed.
- S. Yadav, V. Srivastava, S. Banerjee, C.-H. Weng and Y. C. Sharma, Catena, 2012, 100, 120–127 CrossRef PubMed.
- N. Gupta, A. K. Kushwaha and M. C. Chattopadhyaya, J. Taiwan Inst. Chem. Eng., 2012, 43, 125–131 CAS.
- H.-P. Chao and S.-H. Chen, Chem. Eng. J., 2012, 193–194, 283–289 CrossRef CAS PubMed.
- X. Zou, J. Pan, H. Ou, X. Wang, W. Guan, C. Li, Y. Yan and Y. Duan, Chem. Eng. J., 2011, 167, 112–121 CrossRef CAS PubMed.
- M. M. Abou-Mesalam, Colloids Surf., A, 2003, 225, 85–94 CrossRef CAS.
- M. M. Abou-Mesalam, Adsorption, 2004, 10, 87–92 CrossRef CAS.
- S. Rengaraj and S.-H. Moon, Water Res., 2002, 36, 1783–1793 CrossRef CAS.
- A. F. Shaaban, D. A. Fadel, A. A. Mahmoud, M. A. Elkomy and S. M. Elbahy, J. Environ. Chem. Eng., 2013, 1, 208–217 CrossRef CAS PubMed.
- L. Ćurković, Š. Cerjan-Stefanović and A. Rastovèan-mioè, Water Res., 2001, 35, 3436–3440 CrossRef.
- H. Genç-Fuhrman, J. S. Tjell and D. Mcconchie, Environ. Sci. Technol., 2004, 38, 2428–2434 CrossRef.
- V. K. Gupta, A. Rastogi and A. Nayak, J. Colloid Interface Sci., 2010, 342, 135–141 CrossRef CAS PubMed.
- N. Atar, A. Olgun and S. Wang, Chem. Eng. J., 2012, 192, 1–7 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- S. Kundu and A. K. Gupta, Chem. Eng. J., 2006, 122, 93–106 CrossRef CAS PubMed.
- A. B. Pérez-Marín, V. Meseguer Zapata, J. F. Ortuno, M. Aguilar, J. Sáez and M. Llorens, J. Hazard. Mater., 2007, 139, 122–131 CrossRef PubMed.
- R. A. K. Rao and M. Kashifuddin, Arabian J. Chem., 2012 DOI:10.1016/j.arabjc.2012.01.010 , article in press.
- M. H. Al-Qunaibit, W. K. Mekhemer and A. A. Zaghloul, J. Colloid Interface Sci., 2005, 283, 316–321 CrossRef CAS PubMed.
- J. A. Hefne, W. K. Mekhemer, N. M. Alandis, O. A. Aldayel and T. Alajyan, Int. J. Phys. Sci., 2008, 3, 281–288 Search PubMed.
- L. Zhi-rong and Z. Shao-qi, Process Saf. Environ. Prot., 2010, 88, 62–66 CrossRef PubMed.
- C.-H. Weng, C.-Z. Tsai, S.-H. Chu and Y. C. Sharma, Sep. Purif. Technol., 2007, 54, 187–197 CrossRef CAS PubMed.
- C.-H. Weng, Y. C. Sharma and S.-H. Chu, J. Hazard. Mater., 2008, 155, 65–75 CrossRef CAS PubMed.
- M. G. A. Vieira, A. F. A. Neto, M. L. Gimenes and M. G. C. da Silva, J. Hazard. Mater., 2010, 177, 362–371 CrossRef CAS PubMed.
- S. Kocaoba, Desalination, 2009, 244, 24–30 CrossRef CAS PubMed.
- Y. Liu, D. Xiao and H. Li, Sep. Sci. Technol., 2007, 42, 185–202 CrossRef CAS.
- A. A. El-Bayaa, N. A. Badawy and E. A. AlKhalik, J. Hazard. Mater., 2009, 170, 1204–1209 CrossRef CAS PubMed.
- D. L. Guerra and C. Airoldi, J. Braz. Chem. Soc., 2009, 20, 19–30 CrossRef CAS PubMed.
- M. Eloussaief and M. Benzina, J. Hazard. Mater., 2010, 178, 753–757 CrossRef CAS PubMed.
- A. P. Singh, K. K. Srivastava and H. Shekear, Indian J. Chem. Technol., 2009, 16, 136–141 CAS.
- R. Yu, S. Wang, D. Wang, J. Ke, X. Xing, N. Kumada and N. Kinomura, Catal. Today, 2008, 139, 135–139 CrossRef CAS PubMed.
- D. L. Guerra, C. Airoldi, V. P. Lemos and R. S. Angélica, J. Hazard. Mater., 2008, 155, 230–242 CrossRef CAS PubMed.
- T. S. Anirudhan, C. D. Bringle and P. G. Radhakrishnan, Chem. Eng. J., 2012, 200–202, 149–157 CrossRef CAS PubMed.
- B. I. Olu-Owolabi and E. I. Unuabonah, Appl. Clay Sci., 2011, 51, 170–173 CrossRef CAS PubMed.
- M. G. Mostafa, Y.-H. Chen, J.-S. Jean, C.-C. Liu and Y.-C. Lee, J. Hazard. Mater., 2011, 187, 89–95 CrossRef CAS PubMed.
- M. Al-Harahsheh, R. Shawabkeh, A. Al-Harahsheh, K. Tarawneh and M. M. Batiha, Appl. Surf. Sci., 2009, 255, 8098–8103 CrossRef CAS PubMed.
- L. Yun, W. U. Pingxiao, D. Zhi and Y. E. Daiqi, Acta Geol. Sin., 2006, 80, 219–225 CrossRef PubMed.
- K. G. Bhattacharyya and S. Sen Gupta, Appl. Clay Sci., 2009, 46, 216–221 CrossRef CAS PubMed.
- B. Sarkar, Y. Xi, M. Megharaj, G. S. R. Krishnamurti, D. Rajarathnam and R. Naidu, J. Hazard. Mater., 2010, 183, 87–97 CrossRef CAS PubMed.
- D. L. Guerra, H. C. P. Oliveira, P. C. C. da Costa, R. R. Vian and C. Airoldi, Catena, 2010, 82, 35–44 CrossRef CAS PubMed.
- D. L. Guerra and C. Airoldi, J. Solid State Chem., 2008, 181, 2507–2515 CrossRef CAS PubMed.
- T. S. Anirudhan and P. S. Suchithra, Chem. Eng. J., 2010, 156, 146–156 CrossRef CAS PubMed.
- R. Huang, B. Wang, B. Yang, D. Zheng and Z. Zhang, Desalination, 2011, 280, 297–304 CrossRef CAS PubMed.
- S. Dultz, J.-H. An and B. Riebe, Appl. Clay Sci., 2012, 67–68, 125–133 CrossRef CAS PubMed.
- S.-H. Lin and R.-S. Juang, J. Hazard. Mater., 2002, 92, 315–326 CrossRef CAS.
- S.-Z. Li and P.-X. Wu, J. Hazard. Mater., 2010, 173, 62–70 CrossRef CAS PubMed.
- D. L. Guerra, R. R. Viana and C. Airoldi, Mater. Res. Bull., 2009, 44, 485–491 CrossRef CAS PubMed.
- G. Balomenou, P. Stathi, A. Enotiadis, D. Gournis and Y. Deligiannakis, J. Colloid Interface Sci., 2008, 325, 74–83 CrossRef CAS PubMed.
- D. L. Guerra, V. P. Lemos, R. S. Angélica and C. Airoldi, Colloids Surf., A, 2008, 322, 79–86 CrossRef CAS PubMed.
- C. M. Futalan, C. C. Kan, M. L. Dalida, K.-J. Hsien, C. Pascua and M.-W. Wan, Carbohydr. Polym., 2011, 83, 528–536 CrossRef CAS PubMed.
- G. Zhao, H. Zhang, Q. Fan, X. Ren, J. Li, Y. Chen and X. Wang, J. Hazard. Mater., 2010, 173, 661–668 CrossRef CAS PubMed.
- D.-W. Cho, B.-H. Jeon, C.-M. Chon, Y. Kim, F. W. Schwartz, E.-S. Lee and H. Song, Chem. Eng. J., 2012, 200–202, 654–662 CrossRef CAS PubMed.
- D. J. L. Guerra, J. Goco, J. Nascimento and I. Melo, J. Saudi Chem. Soc., 2013 DOI:10.1016/j.jscs.2013.03.008 , article in press.
- M. Šljivić, I. Smičiklas, S. Pejanović and I. Plećaš, Appl. Clay Sci., 2009, 43, 33–40 CrossRef PubMed.
- N. Bektaş, S. Aydın and M. S. Öncel, Sep. Sci. Technol., 2011, 46, 1005–1016 CrossRef.
- E. Erdem, N. Karapinar and R. Donat, J. Colloid Interface Sci., 2004, 280, 309–314 CrossRef CAS PubMed.
- N. Bektaş and S. Kara, Sep. Purif. Technol., 2004, 39, 189–200 CrossRef PubMed.
- S. Çoruh, Desalination, 2008, 225, 41–57 CrossRef PubMed.
- W. Qiu and Y. Zheng, Chem. Eng. J., 2009, 145, 483–488 CrossRef CAS PubMed.
- X. S. Wang, L. He, H. Q. Hu and J. Wang, Sep. Sci. Technol., 2008, 43, 908 CrossRef CAS.
- E. A. Shukla, E. Johan, T. Henmi and N. Matsue, Procedia Environ. Sci., 2013, 17, 279–284 CrossRef CAS PubMed.
- R. Leyva-Ramos, A. Jacobo-Azuara, P. E. Diaz-Flores, R. M. Guerrero-Coronado, J. Mendoza-Barron and M. S. Berber-Mendoza, Colloids Surf., A, 2008, 330, 35–41 CrossRef CAS PubMed.
- M.-J. Yu, X. Li and W.-S. Ahn, Microporous Mesoporous Mater., 2008, 113, 197–203 CrossRef CAS PubMed.
- Y. J. O. Asencios and M. R. Sun-Kou, Appl. Surf. Sci., 2012, 258, 10002–10011 CrossRef CAS PubMed.
- V. Kailasam, E. Rosenberg and D. Nielsen, Ind. Eng. Chem. Res., 2009, 48, 3991–4001 CrossRef CAS.
- M. Ghoul, M. Bacquet and M. Morcellet, Water Res., 2003, 37, 729–734 CrossRef CAS.
- N. Chiron, R. Guilet and E. Deydier, Water Res., 2003, 37, 3079–3086 CrossRef CAS.
- R. Qu, Y. Zhang, W. Qu, C. Sun, J. Chen, Y. Ping, H. Chen and Y. Niu, Chem. Eng. J., 2013, 219, 51–61 CrossRef CAS PubMed.
- A. Naeem, M. T. Saddique, S. Mustafa, S. Tasleem, K. H. Shah and M. Waseem, J. Hazard. Mater., 2009, 172, 124–128 CrossRef CAS PubMed.
- X. Guo, J. Lu and L. Zhang, J. Taiwan Inst. Chem. Eng., 2013, 44, 630–636 CrossRef CAS PubMed.
- Y.-J. Tu, C.-F. You, C.-K. Chang, S.-L. Wang and T.-S. Chan, Chem. Eng. J., 2012, 198–199, 440–448 CrossRef CAS PubMed.
- S. Mustafa, M. Waseem, A. Naeem, K. H. Shah and T. Ahmad, Desalination, 2010, 255, 148–153 CrossRef CAS PubMed.
- S. Mustafa, M. Waseem, A. Naeem, K. H. Shah, T. Ahmad and S. Y. Hussain, Chem. Eng. J., 2010, 157, 18–24 CrossRef CAS PubMed.
- T. Mahmood, S. U. Din, A. Naeem, S. Mustafa, M. Waseem and M. Hamayun, Chem. Eng. J., 2012, 192, 90–98 CrossRef CAS PubMed.
- Y. Zhang, M. Yang, X.-M. Dou, H. He and D.-S. Wang, Environ. Sci. Technol., 2005, 39, 7246–7253 CrossRef CAS.
- X. Tang, Z. Li, Y. Chen and Z. Wang, Desalination, 2009, 249, 49–57 CrossRef CAS PubMed.
- Y. T. Liu, M. K. Wang, T. Y. Chen, P. N. Chiang, P. M. Huang and J. F. Lee, Environ. Sci. Technol., 2006, 40, 7784–7789 CrossRef CAS.
- S. Milicevic, T. Boljanac, S. Martinovic, M. Vlahovic, V. Milosevic and B. Babic, Fuel Process. Technol., 2012, 95, 1–7 CrossRef CAS PubMed.
- A. B. Albadarin, C. Mangwandi, A. H. Al-Muhtaseb, G. M. Walker, S. J. Allen and M. N. M. Ahmad, Chem. Eng. J., 2012, 179, 193–202 CrossRef CAS PubMed.
- M. Prasad, S. Saxena and S. S. Amritphale, Ind. Eng. Chem. Res., 2002, 41, 105–111 CrossRef CAS.
- Q. Fan, Z. Li, H. Zhao, Z. Jia, J. Xu and W. Wu, Appl. Clay Sci., 2009, 45, 111–116 CrossRef CAS PubMed.
- A. Maiti, S. DasGupta, J. K. Basu and S. De, Ind. Eng. Chem. Res., 2008, 47, 1620–1629 CrossRef CAS.
- M. I. Kandah, Sep. Purif. Technol., 2004, 35, 61–70 CrossRef CAS.
- Z. Zhu, L. Li, H. Zhang, Y. Qiu and J. Zhao, Sep. Sci. Technol., 2010, 45, 262–268 CrossRef CAS.
- J. Giménez, M. Martínez, J. de Pablo, M. Rovira and L. Duro, J. Hazard. Mater., 2007, 141, 575–580 CrossRef PubMed.
- Y. Chang, H. Liu, F. Zha, H. Chen, X. Ren and Z. Lei, Chem. Eng. J., 2011, 167, 183–189 CrossRef CAS PubMed.
- N. Gupta, A. K. Kushwaha and M. C. Chattopadhyaya, J. Taiwan Inst. Chem. Eng., 2012, 43, 125–131 CAS.
- H. Cui, Y. Qian, Q. Li, Q. Zhang and J. Zhai, Chem. Eng. J., 2012, 211–212, 216–223 CrossRef CAS PubMed.
- Y. Huang, X. Ma, G. Liang, Y. Yan and S. Wang, Chem. Eng. J., 2008, 138, 187–193 CrossRef CAS PubMed.
- V. K. Gupta, V. K. Saini and N. Jain, J. Colloid Interface Sci., 2005, 288, 55–60 CrossRef CAS PubMed.
- S. R. Kanel, B. Manning, L. Charlet and H. Choi, Environ. Sci. Technol., 2005, 39, 1291–1298 CrossRef CAS.
- H. K. Boparai, M. Joseph and D. M. O'Carroll, J. Hazard. Mater., 2011, 186, 458–465 CrossRef CAS PubMed.
- P. Yuan, M. Fan, D. Yang, H. He, D. Liu, A. Yuan, J. X. Zhu and T. H. Chen, J. Hazard. Mater., 2009, 166, 821–829 CrossRef CAS PubMed.
- P. Yuan, D. Liu, M. Fan, D. Yang, R. Zhu, F. Ge, J. X. Zhu and H. He, J. Hazard. Mater., 2010, 173, 614–621 CrossRef CAS PubMed.
- Y.-C. Chen, S.-L. Lo and J. Kuo, Colloids Surf., A, 2010, 361, 126–131 CrossRef CAS PubMed.
- S. Maity, S. Chakravarty, S. Bhattacharjee and B. C. Roy, Water Res., 2005, 39, 2579–2590 CrossRef CAS PubMed.
- T. Shi, S. Jia, Y. Chen, Y. Wen, C. Du, H. Guo and Z. Wang, J. Hazard. Mater., 2009, 169, 838–846 CrossRef CAS PubMed.
- E. Roth, V. Mancier and B. Fabre, Geoderma, 2012, 189–190, 133–143 CrossRef CAS PubMed.
- T. W. Cheng, M. L. Lee, M. S. Ko, T. H. Ueng and S. F. Yang, Appl. Clay Sci., 2012, 56, 90–96 CrossRef CAS PubMed.
- S. E. Ghazy and A. H. Ragab, Indian J. Chem. Technol., 2007, 14, 507–514 CAS.
- S. E. Ghazy and A. H. M. Gad, Indian J. Chem. Technol., 2008, 15, 433–442 CAS.
- N. Chandra, N. Agnihotri, P. Sharma, S. Bhasin and S. S. Amritphale, J. Sci. Ind. Res., 2005, 64, 674–678 CAS.
- V. K. Gupta and I. Ali, J. Colloid Interface Sci., 2004, 271, 321–328 CrossRef CAS PubMed.
- V. K. Gupta and I. Ali, Sep. Purif. Technol., 2000, 18, 131–140 CrossRef CAS.
- V. K. Gupta, C. K. Jain, I. Ali, M. Sharma and V. K. Saini, Water Res., 2003, 37, 4038–4044 CrossRef CAS.
- K. Nath, J. P. Gor, P. N. Shah and K. Modi, J. Sci. Ind. Res., 2007, 66, 170–177 CAS.
- L. Tofan, C. Paduraru, D. Bilba and M. Rotariu, J. Hazard. Mater., 2008, 156, 1–8 CrossRef CAS PubMed.
- B. Bayat, J. Hazard. Mater., 2002, 95, 275–290 CrossRef CAS.
- S. S. Banerjee, M. V. Joshi and R. V. Jayaram, Sep. Sci. Technol., 2004, 39, 1611–1629 CrossRef CAS PubMed.
- M. Visa, Appl. Surf. Sci., 2012, 263, 753–762 CrossRef CAS PubMed.
- V. K. Gupta, M. Gupta and S. Sharma, Water Res., 2001, 35, 1125–1134 CrossRef CAS.
- A. Bhatnagar, A. K. Jain, A. K. Minocha and S. Singh, Sep. Sci. Technol., 2006, 41, 1881–1892 CrossRef CAS.
- A. K. Bhattacharya, T. K. Naiya, S. N. Mandal and S. K. Das, Chem. Eng. J., 2008, 137, 529–541 CAS.
- T. K. Naiya, A. K. Bhattacharya and S. K. Das, J. Colloid Interface Sci., 2008, 325, 48–56 CrossRef CAS PubMed.
- S.-C. Pan, C.-C. Lin and D.-H. Tseng, Resour., Conserv. Recycl., 2003, 39, 79–90 CrossRef.
- M. M. Dubinin and L. V. Radushkevich, The equation of the characteristic curve of activated charcoal, Proc. Acad. Sci. USSR, Phys. Chem. Sect., 1947, 55, 331–333 Search PubMed.
- M. Polanyi, Trans. Faraday Soc., 1932, 28, 316–333 RSC.
- S. M. Hasany and R. Ahmad, Sep. Sci. Technol., 2004, 39, 3509–3525 CrossRef CAS PubMed.
- J. P. Hobson, J. Phys. Chem., 1969, 73, 2720–2727 CrossRef CAS.
- F. Helfferich, Ion exchange, McGraw-Hill, New York, 1962 Search PubMed.
- M. S. Onyango, Y. Kojima, O. Aoyi, E. C. Bernardo and H. Matsuda, J. Colloid Interface Sci., 2004, 279, 341–350 CrossRef CAS PubMed.
- A. Ozcan, E. M. Oncu and A. S. Ozcan, Colloids Surf., A, 2006, 277, 90–97 CrossRef PubMed.
- X.-S. Wang, J. Wang and C. Sun, Adsorpt. Sci. Technol., 2006, 24, 517–530 CrossRef CAS.
- A. Sari and M. Tuzen, Sep. Sci. Technol., 2008, 43, 3563–3581 CrossRef CAS.
- J. U. K. Oubagaranadin and Z. V. P. Murthy, Ind. Eng. Chem. Res., 2009, 48, 10627–10636 CrossRef CAS.
- J. U. K. Oubagaranadin and Z. V. P. Murthy, Appl. Clay Sci., 2010, 50, 409–413 CrossRef CAS PubMed.
- J. Perić, M. Trgo and N. V. Medvidović, Water Res., 2004, 38, 1893–1899 CrossRef PubMed.
- H. Ramadan, A. Ghanem and H. El-Rassy, Chem. Eng. J., 2010, 159, 107–115 CrossRef CAS PubMed.
- S. Debnath and U. C. Ghosh, J. Chem. Thermodyn., 2008, 40, 67–77 CrossRef CAS PubMed.
- N. Caliskan, A. R. Kul, S. Alkan, E. G. Sogut and İ. Alacabey, J. Hazard. Mater., 2011, 193, 27–36 CrossRef CAS PubMed.
- O. Redlich and D. L. Peterson, J. Phys. Chem., 1959, 63, 1024 CrossRef CAS.
- Y. C. Wong, Y. S. Szeto, W. H. Cheung and G. McKay, Adsorption, 2008, 14, 11–20 CrossRef CAS.
- S. Debnath and U. C. Ghosh, Chem. Eng. J., 2009, 152, 480–491 CrossRef CAS PubMed.
- S. Rengaraj, J.-W. Yeon, Y. Kim and W.-H. Kim, Ind. Eng. Chem. Res., 2007, 46, 2834–2842 CrossRef CAS.
- M. I. Tempkin and V. Pyzhev, Acta Phys. Chim. USSR, 1940, 12, 327–356 Search PubMed.
- C. Aharoni and M. Ungarish, J. Chem. Soc., Faraday Trans. 1, 1977, 73, 456–464 RSC.
- Y. S. Ho, J. F. Porter and G. McKay, Water, Air, Soil Pollut., 2002, 141, 1–33 CrossRef CAS.
- A. Ahmad, M. Rafatullah, O. Sulaiman, M. H. Ibrahim and R. Hashim, J. Hazard. Mater., 2009, 170, 357–365 CrossRef CAS PubMed.
- Y. Kim, C. Kim, I. Choi, S. Rengraj and J. Yi, Environ. Sci. Technol., 2004, 38, 924–931 CrossRef CAS.
- J. Toth, Acta Chim. Acad. Sci. Hung., 1962, 15, 415–430 Search PubMed.
- T. Düren, Y.-S. Bae and R. Q. Snurr, Chem. Soc. Rev., 2009, 38, 1237–1247 RSC.
- A. Kremleva, S. Krüger and N. Rösch, Geochim. Cosmochim. Acta, 2011, 75, 706–718 CrossRef CAS PubMed.
- P. Mignon, P. Ugliengo, M. Sodupe and E. R. Hernandez, Phys. Chem. Chem. Phys., 2010, 12, 688–697 RSC.
- C. L. Peacock and D. M. Sherman, Geochim. Cosmochim. Acta, 2004, 68, 1723–1733 CrossRef CAS PubMed.
- C. L. Peacock and D. M. Sherman, Geochim. Cosmochim. Acta, 2004, 68, 2623–2637 CrossRef CAS PubMed.
- H. M. Steele, K. Wright, M. A. Nygren and I. H. Hillier, Geochim. Cosmochim. Acta, 2000, 64, 257–262 CrossRef CAS.
- X. Dou, Y. Zhang, B. Zhao, X. Wu, Z. Wu and M. Yang, Colloids Surf., A, 2011, 379, 109–115 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |


