The Baylis–Hillman acetates in organic synthesis: Unprecedented sodium nitrite induced intramolecular Friedel–Crafts cyclization of secondary nitro compounds†
Abstract
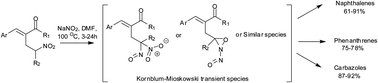
Unprecedented sodium nitrite mediated intramolecular Friedel–Crafts cyclization of alkyl (E)-2-arylidene-4-nitroalkanoates and (E)-3-arylidene-5-nitroalkan-2-ones derived from Baylis–Hillman acetates, providing a facile protocol for synthesis of naphthalenes, phenanthrenes, and carbazoles has been described.

 Please wait while we load your content...
Please wait while we load your content...