DOI:
10.1039/C4RA03563A
(Paper)
RSC Adv., 2014,
4, 31353-31361
In2O3 cubes: synthesis, characterization and photocatalytic properties†
Received
19th April 2014
, Accepted 7th July 2014
First published on 7th July 2014
Abstract
3D cubic microporous In2O3 has been successfully obtained by calcining the as-synthesized cube In(OH)3–InOOH precursor at 300 °C for 2 hours. X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) were employed to clarify the structures and morphologies of both the cubic In(OH)3–InOOH precursor and cubic In2O3. The formation mechanisms of the In(OH)3–InOOH precursor and cubic In2O3 were investigated. As an important semiconductor photocatalytic material, its photocatalytic properties have been tested. Under the irradiation of UV light, the cubic microporous In2O3 exhibits excellent photocatalytic properties to degrade eosin B (EB), which presents ∼95% degradation of EB after 3 hours and the degradation rates is 10.5 times that of commercial In2O3 powder. The high separation efficiency of electron–hole pairs results in high photocatalytic activity. Furthermore, the photoluminescent properties of the cubic microporous In2O3 have been investigated as well.
1. Introduction
A large number of manufactured dyes have been released into the water environment with the development of industry.1,2 Most dyes are resistant to biodegradation, and N-containing dyes undergo anaerobic degradation to yield potentially carcinogenic aromatic amines.3 Therefore, a hot research activity has been devoted recently to the search for semiconductor photocatalysts that directly degrade dyes in wastewater.4,5 The involved photocatalysts, are mostly based on metal oxide semiconductors, such as Bi2O3, Nb2O5, Ag3PO4 etc.6–9 As one of the most important semiconductor materials, indium oxide (In2O3) has been considered as a promising alternative in photocatalysis with the narrower band gap (∼2.8 eV) as well as which can absorb wider solar spectral range than the popular TiO2 (∼3.2 eV).10–12 Furthermore, owing to its nontoxicity and excellent stability, In2O3 has attracted obvious attention as a alternative photocatalytic material.13
As n-type semiconducting oxide of the III–VI compounds, In2O3 exhibits novel electronic and optical properties.14 Due to the low resistivity, high conductivity, high infrared light reflectivity and abundant defects on the surface,15–17 In2O3 has potential applications in computer touch screens,18 optoelectronic field,19 sensors13,20 and photocatalysis.21 Especially, In2O3 shows superior effect in photocatalysis degradation dyes. Many chemists have made great contributions to this field. Shao et al. reported nanocube In2O3/carbon nanofibers heterostructures with high visible light photocatalytic activity for degenerating RhB.22 Cao and co-workers synthesized hollow In2O3 nanocrystals, which also exhibit excellent photocatalytic activities for degenerating RhB and MB under UV irradiation.23 Obviously, properties of materials can often be improved through the structure design. To meet different requirements, many nano- and micro-sized In2O3 with different morphologies including nanocolumns,24 nanofibers,25 nanorods,26 nanosheets,27 nanowires28 and nanotubes29 had been synthesized in succession in the past decade. These structures not only create intriguing variety of morphology features, but also provide good blocks for developing high photocatalytic performance.
In this article, we report a facile method for the preparation of cube In(OH)3–InOOH precursor under mild conditions without any additives. After the precursor is calcined for 2 hours in air, microporous In2O3 cube structures are successfully obtained. The possible formation mechanism of cube In(OH)3–InOOH precursor and cube microporous In2O3 are rationally proposed. Afterwards, photocatalytic performances of the as-prepared In2O3 are investigated and the cube microporous In2O3 exhibits significantly enhanced photocatalytic activity for degradation of eosin B (EB) molecules under UV light irradiation.
2. Experimental section
2.1 Preparation of cube In(OH)3–InOOH precursor and microporous In2O3 cube
All reagents are of analytic grade and were used without further purification. In a typical synthesis, a mixture of InCl3 (0.2 g) and sodium oxalate (0.2 g) in water was stirred for 30 min, and then sealed in a 30 ml Teflon-lined stainless steel autoclave and heated at 150 °C for 1 h. After the sample was gradually cooled to room temperature, a white precipitate was collected and then washed with distilled water and absolute ethanol, and the sample was kept in absolute ethanol. The In2O3 structure was obtained by annealing the corresponding In(OH)3–InOOH precursor in air at 300 °C for 2 h.
2.2 Material characterization
X-ray diffraction analysis of the samples were carried out by an X-ray diffractometer (XRD, Rigaka D/max2500) with Cu Kα radiation (λ = 1.54056 Å). The morphology of the as-prepared products was characterized by scanning electron microscopy (SEM, Hitachi-530 or JEOLJSM-6700F) and transmission electron microscopy (TEM, JEOL-2010, operating voltage of 200 kV). The Brunauer−Emmett−Teller (BET) specific surface area (SBET) of the sample was analyzed by nitrogen adsorption in a Tristar 3000 nitrogen adsorption apparatus, UV spectra were recorded on a Cary 5000 spectrometer at room temperature. Fluorescence spectrum was characterized by RPM2000.
2.3 Photocatalytic reactions
The photocatalytic activities of the as-prepared microporous In2O3 cube structures were evaluated by the photocatalytic degradation of EB aqueous solution via an XPA-system Photochemical Reactor (Nanjing, China) at room temperature under ultraviolet light irradiation. A 300 W Hg arc lamp was used as a light source to provide the UV light, the light intensity is ∼7 mW cm−2. In a typical reaction, 0.05 g of as-prepared photocatalysts was dispersed into 50 mL of EB aqueous solution (1 × 10−5 M). Before light irradiating, the suspension was stirred for 1 hour in the dark to reach adsorption equilibrium of EB on the surface of cube structures. Then, the reaction was stopped at 1 hour intervals and 10 mL of reaction solutions were extracted to determine the concentrations of the aqueous EB solution by UV-vis spectroscopy. In this study, commercial In2O3 was used as a reference catalyst to photocatalytic EB under the same condition as the as-prepared samples. EB aqueous solution without photocatalysts irradiated by UV light was used as a blank experiment and the as-prepared photocatalysts reacting with EB in dark were used as comparative evaluation.
3. Results and analysis
3.1 Structural analysis of cube In(OH)3–InOOH precursor and corresponding In2O3 structure
The phase structure of the obtained product was first investigated by X-ray diffraction (XRD). Fig. 1 shows the XRD pattern of the as-prepared In(OH)3–InOOH precursor. The XRD pattern indicates that the two types of crystals are the cubic In(OH)3 in space group Im-3 (a = b = c = 7.974 Å, JCPDS: 76-1463) and the orthorhombic InOOH in space group P21nm (a = 5.260 Å, b = 4.560 Å, c = 3.270 Å, JCPDS: 71-2283). The observed peaks of In(OH)3 could be assigned to diffraction from (200), (220), (222), (400), (420) and (422) faces. Meanwhile, the characteristic peaks of InOOH could be correspond to diffraction from the (110), (101), (011), (200), (021) and (121) faces. No impurities could be detected in the curve, suggesting high purity of the as-synthesized In(OH)3–InOOH. The morphology of the resulting In(OH)3–InOOH sample were investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Fig. 2a and b, the typical morphology of the as-prepared complex is cube structure with an average diameter of 600 nm. Fig. 2c shows a typical TEM image of one surface of polyhedron crystal. As shown in the inset in Fig. 2d, the crystalline lattice distances in the white frame are 2.82 Å and 3.44 Å, corresponding to the (220) face of cubic In(OH)3 and (110) face of orthorhombic InOOH, respectively, which are consistent with the XRD results.
 |
| | Fig. 1 XRD pattern of the synthetic In(OH)3–InOOH precursor. | |
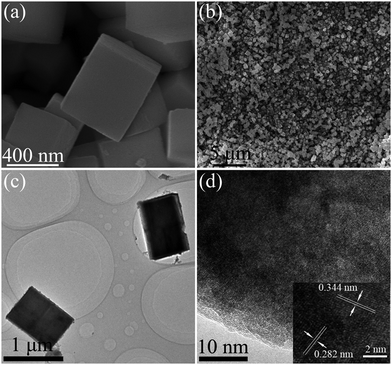 |
| | Fig. 2 SEM images of the synthetic In(OH)3–InOOH precursor: (a) high magnification, (b) low magnification. TEM images of the synthetic In(OH)3–InOOH precursor: (c) low magnification, (d) high magnification and HRTEM image (insert) of the In(OH)3–InOOH precursor. | |
Similar to other metal hydroxides,30,31 In(OH)3–InOOH precursor cube can also be converted into In2O3 derivatives upon heating. From the TG-DSC result (Fig. S1†), the calcination temperature was ascertained to be 300 °C. After calcining the as-synthesized cube In(OH)3–InOOH precursor at 300 °C for 2 h in air, microporous In2O3 structure could be successfully obtained. Fig. 3 shows the corresponding XRD patterns of calcined products. All the diffraction peaks in this pattern could be indexed to a single phase of cubic In2O3 in space group la-3, which matches well with JCPDS: 06-0416 (a = b = c = 10.118 Å). As shown in Fig. 4a and b, the morphologies of the In(OH)3–InOOH precursor were well transmitted to the product In2O3 besides pores structures, which indicates that the solid-state transformation of In2O3 from In(OH)3–InOOH precursor by thermally decomposing is a topotactic process. In addition, the sample was characterized by TEM (Fig. 4c), and the low-magnification TEM images further clearly indicate that the sample has similar morphologies to the hydroxide precursors. However, many pores have been clearly observed inside the cube due to release of H2O in the process of the decomposition of In(OH)3–InOOH precursor. The HR-TEM images of an individual In2O3 cube was shown in Fig. 4d, which reveals that the crystalline lattice distances in the white frame are 4.13 Å, corresponding to the (211) crystalline plane of In2O3.
 |
| | Fig. 3 XRD pattern of the synthetic cube In2O3. | |
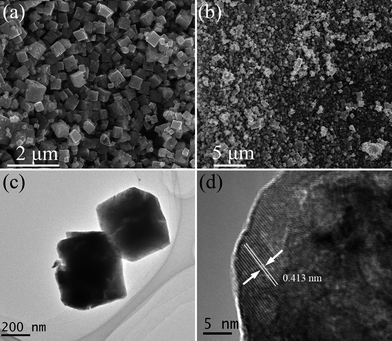 |
| | Fig. 4 SEM images of the cube In2O3: (a) high magnification, (b) low magnification. TEM images of the cube In2O3: (c) low magnification, (d) HRTEM image. | |
BET gas sorptometry measurements were conducted to examine the porous nature of the In2O3 cube. As shown in Fig. 5, the N2 adsorption/desorption isotherm of the sample was identified as type II isotherm based on Brunauer–Deming–Deming–Teller (BDDT) classification, and the saturation adsorption platform at low P/P0 indicated the presence of micropores in the sample. The distribution of pore size indicates that a majority of the pores are smaller than 5 nm. These porous probably appeared among the surface of In2O3 cube and the gap by release of H2O. The BET surface area reaches 71.41 m2 g−1.
 |
| | Fig. 5 N2 adsorption/desorption isotherm and Barrett–Joyner–Halenda (BJH) pore size distribution plot (inset) of In2O3. | |
3.2 Possible formation mechanisms of cube In(OH)3–InOOH precursor and In2O3 structure
It is well known that the final morphology of crystal was controlled by the nature of crystal structure during the one stage of nucleation and the other stage of Ostwald ripening through the delicate affect of external factors, examples of the additive, concentration, reaction time and so on.32–34 Therefore, the final morphology of In(OH)3–InOOH precursor are determined by the combination of internal and external factors.
Firstly, due to the process of synthesis without any organic additives or templates, the morphology of the final product is largely determined by the natural structure of In(OH)3 and InOOH. To the best of our knowledge, In(OH)3 crystallizes in cubic space group Im-3 and InOOH in orthorhombic space group P21nm. As demonstrated by Fig. 6c and d, both In(OH)3 and InOOH crystallizes in distort cube structure. The fundamental building unit of In(OH)3 and InOOH consists of In8O12 (Fig. 6a) and In8O8 (Fig. 6b) hexahedral structure, respectively, and each In8O12 or In8O8 hexahedral structure sharing six planar quadrangle windows with six neighbors to generate the resulting 3D cube framework, which was also supported by the SEM and TEM observations. In addition, from the perspective of topology, each In atom connects six adjacent In atoms by O atoms and serves as a 6-connected node. Thus, the overall network of In(OH)3–InOOH precursor can be described as a 6-connected pcu topology (Fig. 6e) with the Schläfli symbol of 41263, which is a classic 3D cube structure topology.35–37 Consequently, the cube In(OH)3–InOOH precursor along the a, b, c-axis in space are thermodynamically stable, and thus readily tends to form cube in shape due to their intrinsic hexahedral structure.
 |
| | Fig. 6 (a) A schematic view of fundamental building unit of In(OH)3 with In8O12 hexahedral structure and (b) In8O8 hexahedral structure of InOOH; (c) perspective view of 3D framework of In(OH)3 and (d) InOOH; (e) schematic representation of pcu topology with the Schläfli symbol of 41263. | |
Secondly, when we focused essentially on the influence by external factors, we found that the final morphology and size of In(OH)3–InOOH precursor were strongly correlated to alkaline sources used. We conducted the alkaline-dependent experiments by changing different alkaline sources while the other experimental conditions unchanged. There is no regular product collected if sodium oxalate is replaced by ethylenediamine (Fig. 7a). Meanwhile, when NaOH or hexamethylenetetramine were used as alkaline sources, irregular In(OH)3–InOOH precursor were produced (Fig. 7b and c). Only when sodium oxalate was used as alkaline sources, the cube In(OH)3–InOOH precursor with diameters of 600 nm was produced. To disclose the formation mechanism of In(OH)3–InOOH precursor, further experiments exploring the effect of synthetic temperature on the formation of the morphology and size of In(OH)3–InOOH precursor were investigated. The related results are shown in Fig. S2.†
 |
| | Fig. 7 SEM images of the In(OH)3–InOOH precursor prepared using different alkaline sources (a) ethylenediamine, (b) NaOH, (c) hexamethylenetetramine. | |
On the basis of the above results, a growth process of the cube In(OH)3–InOOH precursor and In2O3 may be illustrated as followed:
| | |
CH3COO− + H2O ↔ CH3COOH + OH−
| (1) |
| | |
In3+ + 3OH− → In(OH)3/InOOH
| (2) |
| | |
In(OH)3/InOOH → In2O3
| (3) |
Firstly, the hydrolysis of the sodium oxalate results in the formation of OH− ions, which provide a weak basic environment for the formation of indium hydroxide crystal nucleuses. Subsequently, because of its nature cube structure, the crystal nucleuses of indium hydroxide gradually grow into uniformly large cube due to the Ostwald ripening to reduce surface area and decrease the energy.
3.3 Optical properties
The UV-vis diffuse reflectance spectra of cube In2O3 product is shown in Fig. 8, a strong absorption can be observed in the ultraviolet region. According to the plot of transformed Kubelka–Munk function versus the energy of exciting light, the value of the band gap for the cube In2O3 was estimated to be 2.7 eV.
 |
| | Fig. 8 UV-vis diffuse reflectance of In2O3; the inset in the upper right is the absorption1/2 versus energy curve. | |
To investigate optical property of the sample, we measured the emission and excitation spectra shown in Fig. 9a and b, in which Fig. 9a is the emission spectrum of particles excited by 315 nm light. It can be found that the sample show wide-band emission in white range with a peak plat around 475 nm. It can be probably used as white lighting. As mentioned above, cube In2O3 is a semiconductor with an energy band Eg = 2.7 eV. When illuminated by 315 nm (about 3.95 eV in energy) light, electrons are excited into conduction band and then jump into valance band with strong emission at 444 nm (corresponding to 2.7 eV in energy) if just only taking band edge transition into consideration. The peak flat of emission at 475 nm is a slight red-shift phenomenon that is widely observed for nano-particles. However, due to that the excited energy is much larger than 2.7 eV, the electrons are excited to higher levels than bottom of conduction band (−0.63 eV versus NHE), the emission spectrum are much complex including inter-band jumping from higher level (electrons) to higher levels (holes), intra-band jumping from higher level (electrons) to bottom of conducting band, etc. This is the reason for the white emission. Fig. 9b is the excitation spectrum of prepared In2O3 particles. There are many two peaks locating at 315 nm and 365 nm. The two absorption peaks may arise from the quantum transitions happening between the energy level of In3+ ions.
 |
| | Fig. 9 The emission (a) and excitation spectra (b) of cube In2O3. | |
3.4 Application in photogradation of eosin B (EB)
Eosin B has been successfully used in surface-second harmonic generation studies of acid–base equilibrium,38 and orientational relaxation at air/water interfaces, which belongs to xanthene family dye.39 We assessed the potential validity of cube In2O3 for photocatalytic removal of EB. As a comparison, the EB photodegraded by commercial In2O3 was also performed. The characteristic absorption peak of EB at λ = 514 nm was selected as monitoring wave length. Control experiments revealed that the photodegradation of EB irradiated under UV light could almost be neglected in the absence of cube In2O3. Also, there was no appreciable degradation of EB over the cube In2O3 after 5 h in the absence of UV light irradiation. As observed in Fig. 10a, with the increase of illumination time, the main absorption peak of EB gradually weakened. No other absorption bands appear in either the ultraviolet or visible region. After 3 h irradiation under UV light, the photodegradation ratio of EB was about 95% (Fig. 10b). However, the photodegradation ratio of EB was only 33% in the presence of commercial In2O3 after 5 h. Obviously, the as-prepared In2O3 exhibits better photocatalytic activity than commercial In2O3. This is mostly ascribe to the larger BET surface area (cube In2O3: 71.41 m2 g−1; commercial In2O3: 20.33 m2 g−1), which leads to more surface reaction sites.23,40 In particular, the microporous structure of the catalyst facilitates light waves to penetrate deep inside the photocatalyst and beget high mobility of charge, which also leads to higher catalytic activity.41–43 Due to the low initial concentrations of the reactants (the initial concentration (c0) = 10 mg L−1 for EB in the present experiment), the photocatalytic degradation of EB over the cube In2O3 follows pseudo-first-order based on the kinetic linear simulation,44 that can be fitted to the equation:| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c0/c = kt c0/c = kt
| (4) |
where c0 is the initial EB concentration, c indicates the EB concentration after irradiation time t, k is the reaction rate constant. In this system, the k for cube In2O3 and commercial In2O3 are kcube In2O3 (1.0447 h−1) and kcommercial In2O3 (0.0991 h−1), respectively, which are also summarized in Fig. 10c.
 |
| | Fig. 10 (a) Absorption spectra of a solution of EB in the presence of cube In2O3 and under exposure to UV light, (b) time course of the decrease in the dye concentration using different catalysts, (c) corresponding selected fitting results using pseudo-first-order reaction kinetics. (d) Cycling runs in the photocatalytic degradation of EB by cube In2O3 products. | |
Moreover, the stability of photocatalysts is also one of most important factor from the practical viewpoint as well as its photocatalytic activity. Thus, recycle experiments were further investigated. After three recycles for the photodegradation of EB, the hierarchical microsphere photocatalysts did not exhibit significant loss of activity (Fig. 10d), indicating that these photocatalysts have relatively high stability during the photocatalytic oxidation of the dye.
3.5 Proposed degradation mechanism of EB
In order to monitor the active radicals that form during the photodegraded process, we first investigated the effect of band structure on the activity of the as-prepared In2O3. We can calculate its conduction and valence band positions through the following equation: ECB = X − EC − 1/2Eg,45 where X is the absolute electronegativity of the semiconductor. EC is the energy of free electrons on the hydrogen scale (∼4.5 eV). Eg is the band gap energy of the semiconductor. As a result, CB of In2O3 is −0.63 eV, which is 0.3 eV lower than that of O2/O2˙− (−0.33 eV), which may facilitate for the O2˙− generated to involve in degradation. The VB is estimated to be 2.07 eV, which is 0.08 eV higher than that of ˙OH/OH− (+1.99 eV), suggesting that the hole photogenerated on the surface of In2O3 could react with OH−/H2O to form ˙OH from the theoretical viewpoint.46 To further confirm the presence of active radicals, the EB/In2O3 system was examined by DMPO spin-trapped ESR spectroscopy. As shown in Fig. 11, there were no DMPO-˙OH or DMPO-O2˙− signal can be detected before irradiation. While after irradiation (15 min), characteristic four peaks of the DMPO-˙OH adduct with intensity 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were observed. However, when the irradiation time prolong to 30 min, the ESR signals disappeared. As for O2˙−, due to its unstable in aqueous solution and is easily decomposed into ˙OH, we used methanol as solvent instead of water. It is shown in Fig. 11b that O2˙− signals were observed in the UV illuminated methanol solution of In2O3/EB/DMPO, confirming that O2˙− also generated during the In2O3 photodegradation of EB.
1 were observed. However, when the irradiation time prolong to 30 min, the ESR signals disappeared. As for O2˙−, due to its unstable in aqueous solution and is easily decomposed into ˙OH, we used methanol as solvent instead of water. It is shown in Fig. 11b that O2˙− signals were observed in the UV illuminated methanol solution of In2O3/EB/DMPO, confirming that O2˙− also generated during the In2O3 photodegradation of EB.
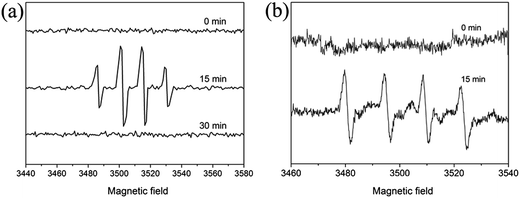 |
| | Fig. 11 (a) EPR spectra of DMPO-˙OH adducts for In2O3/EB dispersion in water, (b) DMPO-O2˙− adducts for In2O3/EB dispersion in methanol. | |
From our experimental results, a proposed degradation mechanism was being discussed to explain the enhancement of the photocatalytic properties of the cube In2O3. According to the classic photocatalytic theory, cube In2O3 generate valence-band holes (h+) and conduction-band electrons (e−) pairs at the surface after absorbing an ultraviolet light energy hv, which is match the band gap energy (Eg) (reaction (5)).47 The h+ could react with hydroperoxy adsorbed on the surface of cube In2O3 to generate highly reactive hydroxyl radicals (˙OH), which could direct reaction of the organic pollutants with surface light energy (reaction (6) and (7)).48 On the other hand, the surface of cube In2O3 e− could react with dissolved oxygen molecules to yield superoxide radical O2˙−. The superoxide radical O2˙− react with water to produce hydroperoxy. Hydroperoxy is a key intermediate product for the latter route, which can easily break down and also provide conditions for subsequent produce ˙OH radicals (reaction (8)–(10)). Therefore, large number of ˙OH radicals is resulting in the enhanced photocatalytic activity.49–51
| | |
OH˙ + h+ + EB → degrade products
| (7) |
| | |
2O2˙− + 2H2O → H2O2 + O2 + 2OH−
| (9) |
| | |
H2O2 + e− →˙OH + OH−
| (10) |
To further validate that the electron–hole pairs were easily separated and transferred to the surface of cube In2O3 under UV irradiation, the electrochemical impendence spectroscopy (EIS) were determined. The EIS spectra were completed using a potential amplitude of 5 mV over a frequency range of 0.1−106 Hz and were simulated using the Zview software. The results (Fig. 12) reveal that the cube In2O3 has higher conductivity than commercial In2O3, that is, in the case of cube In2O3, the photoinduced electron–hole pairs were easily separated and transferred to the sample surface compared with commercial In2O3.52–54 To obtain more detailed information, equivalent circuit can be designed for two kinds of In2O3. From Table S1,† it can be observed that the Rs of cube In2O3 is smaller than the Rs of commercial In2O3, which leads to improved catalytic property of cube In2O3.
 |
| | Fig. 12 Nyquist plots of the dummy cell fabricated with cube In2O3 (a) and commercial In2O3 (b). The equivalent circuit used to fit the EIS of cube In2O3 (c) and commercial In2O3 (d). | |
4. Conclusions
In summary, we prepared microporous In2O3 cube via two-step hydrothermal routes and a subsequent sintering process. The formation mechanism of In(OH)3–InOOH cube precursor and In2O3 cube have been discussed in detail. The In2O3 cube exhibits excellent photocatalytic activities as photocatalyst for degeneration of EB under UV irradiation and its degradation rate is 10.5 times of that of commercial In2O3 powder due to its higher BET surface area. Researches on the photocatalytic mechanism indicate that the ˙OH, O2˙− and holes from the photocatalytic process are responsible together for its high photocatalytic performance. It is expected that this microporous materials will greatly promote their practical application to eliminate the dyes from wastewater.
Acknowledgements
This work was supported by the National Science Foundation of China (no. 21271108), the Ministry of Science and Technology (Grant 2014CB932001), 2011 Science Foundation of Tianjin (no. 11JCZDJC24800), and China–U.S. Center for Environmental Remediation and Sustainable Development.
References
- A. B. Dos Santos, F. J. Cervantes and J. B. van Lier, Bioresour. Technol., 2007, 98, 2369–2385 CrossRef CAS PubMed.
- H. Zollinger, Synthesis, Properties of Organic Dyes and Pigments, VCH Publishers, New York, 1987 Search PubMed.
- S. Horikoshi, F. Hojo and H. Hidaka, Environ. Sci. Technol., 2004, 38, 2198–2208 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- Z. Zou, J. Ye, K. Sayama and H. Arakawa, Nature, 2001, 414, 625–627 CrossRef CAS PubMed.
- M. Ge, Y. F. Li, L. Liu, Z. Zhou and W. Chen, J. Phys. Chem. C, 2011, 115, 5220–5225 CAS.
- N. Kim, R. N. Vannier and C. P. Grey, Chem. Mater., 2005, 17, 1952–1958 CrossRef CAS.
- S. X. Ge, H. M. Jia, H. X. Zhao, Z. Zheng and L. Z. Zhang, J. Mater. Chem., 2010, 20, 3052–3058 RSC.
- M. Ge, N. Zhu, Y. P. Zhao, J. Li and L. Liu, Ind. Eng. Chem. Res., 2012, 51, 5167–5173 CrossRef CAS.
- J. B. Mu, B. Chen, M. Y. Zhang, Z. C. Guo, P. Zhang, Z. Y. Zhang, Y. Y. Sun, C. L. Shao and Y. C. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 424–430 CAS.
- Z. Y. Wang, B. B. Huang, Y. Dai, X. Y. Qin, X. Y. Zhang, P. Wang, H. X. Liu and J. X. Yu, J. Phys. Chem. C, 2009, 113, 4612–4617 CAS.
- J. Lv, T. Kako, Z. S. Li, Z. G. Zou and J. H. Ye, J. Phys. Chem. C, 2010, 114, 6157–6162 CAS.
- X. M. Xu, X. Li, W. B. Wang, B. Wang, P. Sun, Y. F. Sun and G. Y. Lu, RSC Adv., 2014, 4, 4831–4835 RSC.
- C. Q. Wang, D. R. Chen, X. L. Jiao and C. L. Chen, J. Phys. Chem. C, 2007, 111, 13398–13403 CAS.
- Y. Zhang, H. Jia, D. Yu, X. Luo, Z. Zhang and X. Chen, J. Mater. Res., 2003, 18, 2793–2798 CrossRef CAS.
- I. Hamberg and C. G. Granqvist, J. Appl. Phys., 1986, 60, R123–R160 CrossRef CAS PubMed.
- A. Datta, S. K. Panda, D. Ganguli, P. Mishra and S. Chaudhuri, Cryst. Growth Des., 2009, 9, 113–117 Search PubMed.
- S. Parthiban, K. Ramamurthi, E. Elangovan, R. Martins and E. Fortunato, Appl. Phys. Lett., 2009, 94, 212101–212103 CrossRef PubMed.
- H. Cao, X. Qiu, Y. Liang, Q. Zhu and M. Zhao, Appl. Phys. Lett., 2003, 83, 761–763 CrossRef CAS PubMed.
- N. Du, H. Zhang, B. Chen, X. Ma, Z. Liu, J. Wu and D. Yang, Adv. Mater., 2007, 19, 1641–1645 CrossRef CAS.
- B. Li, Y. Xie, M. Jing, G. Rong, Y. Tang and G. Zhang, Langmuir, 2006, 22, 9380–9385 CrossRef CAS PubMed.
- J. B. Mu, C. L. Shao, Z. C. Guo, M. Y. Zhang, Z. Y. Zhang, P. Zhang, B. Chen and Y. C. Liu, J. Mater. Chem., 2012, 22, 1786–1793 RSC.
- J. F. Yin and H. Q. Cao, Inorg. Chem., 2012, 51, 6529–6536 CrossRef CAS PubMed.
- P. Guha, S. Kar and S. Chaudhuri, Appl. Phys. Lett., 2004, 85, 3851–3853 CrossRef CAS PubMed.
- C. H. Liang, G. W. Meng, Y. Lei, F. Phillipp and L. D. Zhang, Adv. Mater., 2001, 13, 1330–1333 CrossRef CAS.
- Z. X. Cheng, X. B. Dong, Q. Y. Pan, J. C. Zhang and X. W. Dong, Mater. Lett., 2006, 60, 3137–3140 CrossRef CAS PubMed.
- X. M. Xu, D. W. Wang, W. B. Wang, P. Sun, J. Ma, X. H. Liang, Y. F. Sun, Y. G. Ma and G. Y. Lu, Sens. Actuators, B, 2012, 171–172, 1066–1072 CrossRef CAS PubMed.
- Y. F. Hao, G. W. Meng, C. H. Ye and L. D. Zhang, Cryst. Growth Des., 2005, 5, 1617–1621 CAS.
- C. L. Chen, D. R. Chen, X. L. Jiao and C. Q. Wang, Chem. Commun., 2006, 4632–4634 RSC.
- D. Li, X. C. Duan, Q. Qin, H. M. Fan and W. J. Zheng, J. Mater. Chem. A, 2013, 1, 12417–12421 CAS.
- S. M. Yuan, J. X. Li, L. T. Yang, L. W. Su, L. Liu and Z. Zhou, ACS Appl. Mater. Interfaces, 2011, 3, 705–709 CAS.
- J. M. Ma, J. Q. Yang, L. F. Jiao, Y. H. Mao, T. H. Wang, X. C. Duan, J. B. Lian and W. J. Zheng, CrystEngComm, 2012, 14, 453–459 RSC.
- J. M. Ma, Y. P. Wang, Y. J. Wang, P. Peng, J. B. Lian, X. C. Duan, Z. F. Liu, X. D. Liu, Q. Chen, T. I. Kim, G. Yao and W. J. Zheng, CrystEngComm, 2011, 13, 2369–2374 RSC.
- J. M. Ma, X. D. Liu, J. B. Lian, X. C. Duan and W. J. Zheng, Cryst. Growth Des., 2010, 10, 2522–2527 CAS.
- E. V. Alexandrov, V. A. Blatov, A. V. Kochetkov and D. M. Proserpio, CrystEngComm, 2011, 13, 3947–3958 RSC.
- O. Delgado-Friedrichs, M. D. Foster, M. O'Keeffe, D. M. Proserpio, M. M. J. Treacy and O. M. Yaghi, J. Solid State Chem., 2005, 178, 2533–2554 CrossRef CAS PubMed.
- I. A. Baburin, V. A. Blatov, L. Carlucci, G. Ciani and D. M. Proserpio, Cryst. Growth Des., 2008, 8, 519–539 CAS.
- A. A. Tamburello-Luca, P. Hebert, R. Antoine, P. F. Brevet and H. H. Girault, Langmuir, 1997, 13, 4428–4434 CrossRef CAS.
- P. Fita, M. Fedoseeva and E. Vauthey, J. Phys. Chem. A, 2011, 115, 2465–2470 CrossRef CAS PubMed.
- L. Zhou, W. Wang, H. Xu, S. Sun and M. Shang, Chem.–Eur. J., 2009, 15, 1776–1782 CrossRef CAS PubMed.
- X. C. Wang, J. C. Yu, C. M. Ho, Y. D. Hou and X. Z. Fu, Langmuir, 2005, 21, 2552–2559 CrossRef CAS PubMed.
- C. S. Guo, M. Ge, L. Liu, G. D. Gao, Y. C. Feng and Y. Q. Wang, Environ. Sci. Technol., 2010, 44, 419–425 CrossRef CAS PubMed.
- L. Z. Zhang and J. C. Yu, Chem. Commun., 2003, 2078–2079 RSC.
- M. Lee, S. Park, G. Lee, C. Ju and S. Hong, Catal. Today, 2005, 101, 283–290 CrossRef CAS PubMed.
- T. B. Li, G. Chen, C. Zhou, Z. Y. Shen, R. C. Jin and J. X. Sun, Dalton Trans., 2011, 40, 6751–6758 RSC.
- H. B. Fu, C. S. Pan, W. Q. Yao and Y. F. Zhu, J. Phys. Chem. B, 2005, 109, 22432–22439 CrossRef CAS PubMed.
- C. S. Pan and Y. F. Zhu, Environ. Sci. Technol., 2010, 44, 5570–5574 CrossRef CAS PubMed.
- S. H. Yoon and J. H. Lee, Environ. Sci. Technol., 2005, 39, 9695–9701 CrossRef CAS.
- M. M. Cheng, W. H. Ma, J. Li, Y. P. Huang, J. C. Zhao, Y. X. Wen and Y. M. Xu, Environ. Sci. Technol., 2004, 38, 1569–1575 CrossRef CAS.
- S. Sato, Langmuir, 1988, 4, 1156–1159 CrossRef CAS.
- S. Q. Guo, X. Zhang, Z. Zhou, G. D. Gao and L. Liu, J. Mater. Chem. A, 2014, 2, 9236–9243 CAS.
- L. Zhang, H. Fu and Y. Zhu, Adv. Funct. Mater., 2008, 18, 2180–2189 CrossRef CAS.
- S. Zhu, T. Xu, H. Fu, J. Zhao and Y. Zhu, Environ. Sci. Technol., 2007, 41, 6234–6239 CrossRef CAS.
- M. Y. Liu, G. Li and X. S. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 2604–2610 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03563a |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c0/c = kt
c0/c = kt
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were observed. However, when the irradiation time prolong to 30 min, the ESR signals disappeared. As for O2˙−, due to its unstable in aqueous solution and is easily decomposed into ˙OH, we used methanol as solvent instead of water. It is shown in Fig. 11b that O2˙− signals were observed in the UV illuminated methanol solution of In2O3/EB/DMPO, confirming that O2˙− also generated during the In2O3 photodegradation of EB.
1 were observed. However, when the irradiation time prolong to 30 min, the ESR signals disappeared. As for O2˙−, due to its unstable in aqueous solution and is easily decomposed into ˙OH, we used methanol as solvent instead of water. It is shown in Fig. 11b that O2˙− signals were observed in the UV illuminated methanol solution of In2O3/EB/DMPO, confirming that O2˙− also generated during the In2O3 photodegradation of EB.








