A mechanistic and kinetic study of the heterogeneous degradation of chlorpyrifos and chlorpyrifos oxon under the influence of atmospheric oxidants: ozone and OH-radicals
Abstract
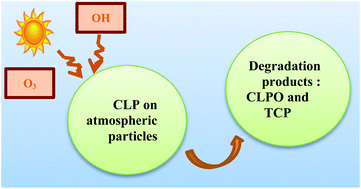
Chlorpyrifos (CLP) is a widely used pesticide and chlorpyrifos oxon (CLPO) is one of its degradation products. They are both detected in the gas and particulate phase of the atmosphere. The kinetics and mechanism of heterogeneous oxidation of CLP and CLPO by ozone and OH radicals have been studied at room temperature using a photochemical reactor coupled to a GC/MS analytical system. CLPO and trichloropyridinol (TCP) were identified as degradation products in sample residues of both ozonolysis and OH-oxidation experiments of CLP. The yields of these products were measured and the results show that pathways producing TCP and CLPO are both of ∼50% for ozonolysis of CLP, whereas OH oxidation produces ∼50% CLPO and ∼25% TCP. Heterogeneous OH-oxidation rates of CLP and CLPO are greater than those measured for ozonolysis, and CLP is twenty times more reactive than CLPO. The calculated CLP lifetimes suggest that once emitted into the atmosphere, CLP can be degraded quite rapidly (∼2 day) by OH radicals in the heterogeneous phase. Meanwhile, CLPO's tropospheric lifetime in the heterogeneous phase towards OH-radicals is in the order of several weeks.

 Please wait while we load your content...
Please wait while we load your content...