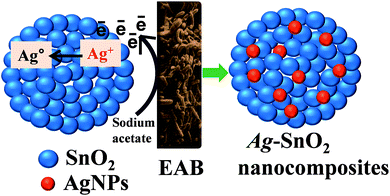
Visible light-driven photocatalytic and photoelectrochemical studies of Ag–SnO2 nanocomposites synthesized using an electrochemically active biofilm†
Sajid Ali Ansari
,
Mohammad Mansoob Khan
,
Mohd Omaish Ansari
,
Jintae Lee
and
Moo Hwan Cho
*
School of Chemical Engineering, Yeungnam University, Gyeongsan-si, Gyeongbuk 712-749, South Korea. E-mail: mhcho@ynu.ac.kr; Fax: +82-53-810-4631; Tel: +82-53-810-2517
First published on 3rd June 2014
Abstract
Ag–SnO2 nanocomposites (1 mM and 3 mM) were synthesized in water at room temperature using an electrochemically active biofilm. The resulting nanocomposites were characterized by X-ray diffraction, transmission electron microscopy, diffuse reflectance spectroscopy, photoluminescence spectroscopy and X-ray photoelectron spectroscopy. The Ag–SnO2 nanocomposites exhibited enhanced photocatalytic activity under visible light irradiation for the degradation of methyl orange, methylene blue, 4-nitrophenol and 2-chlorophenol compared with pure SnO2 nanostructures. Photoelectrochemical measurements, such as electrochemical impedance spectroscopy, linear scan voltammetry and differential pulse voltammetry in the dark and under visible light irradiation, further supported the visible light activity of the Ag–SnO2 nanocomposites. These results showed that the Ag nanoparticles induced visible light activity and facilitated efficient charge separation in the Ag–SnO2 nanocomposites, thereby improving the photocatalytic and photoelectrochemical performance.
Introduction
Metal oxide photocatalysis has attracted significant attention because of its promising applications in various fields, such as environment remediation by the photodecomposition of hazardous dyes in polluted water, industrial effluents and solar energy conversion.1,2 Metal oxides, such as TiO2, ZnO and SnO2, have been the prime choice for basic research and practical applications owing to their high activity, good stability, low cost, non-toxicity and chemical inertness.3–6 One drawback of these metal oxides is their wide band-gap, which is illustrated by their absorption of UV light but not in visible light. Consequently, only small region of the solar spectrum can be utilized when these metal oxides are used as a photocatalyst. Because visible light comprises ∼43% of the solar spectrum, therefore, a photocatalyst that is active under visible light is needed.6–8 Accordingly, considerable efforts has been made to develop visible-light-active metal oxide photocatalysts that can make use of radiation in the visible light region.2–4,6–8 Recently, the synthesis of nanocomposites by combining metal oxides with noble metals has received significant attention.9 The light harvesting properties of noble metals, such as Ag and Au, have been exploited worldwide by most researchers for the photocatalytic reaction under visible light irradiation. Furthermore, the electrons in noble metals are excited by a surface plasmon resonance phenomenon and are transferred to the conduction band of the metal oxide. The transferred electrons move to the surface of the metal oxide materials and assist in the formation of super-oxide radical anions and hydroxyl radicals, whereas the holes present on the surface of the metals can act as redox centers to initiate the photocatalytic reactions and enhance the photocatalytic efficiency of metal oxides.9–15SnO2 has attracted increasing attention as an important metal oxide because of its wide range of applications, such as optoelectronic devices and dye sensitized solar cells, owing to its excellent optical and electrical properties.6,16 SnO2 normally exhibits high photocatalytic activity under UV light irradiation, and considerable efforts have been made to enhance its photoactivity in the visible region.6,16 Better processing methods might improve significantly the catalytic performance of SnO2. Recently, there have been many reports on the synthesis of photoactive noble metal–SnO2 nanocomposites using a range of synthetic methods. Wu et al.17 used a solution-based route for the synthesis of Au–SnO2 hybrid nanostructures, where as You et al.18 reported a solution-based method to prepare Au–SnO2 nanostructures and further tested their photocatalytic activity. These reports have shown that noble metal nanoparticles, such as silver nanoparticles (AgNPs) and gold nanoparticles, anchored to the metal oxide surface enhance the efficiency of the charge-transfer process. On the other hand, most of the methods reported for the synthesis of metal–metal oxide nanocomposite generally use harmful chemicals, whose discharge into the environment affects the biosphere, which might be related to human health issues. Therefore, there is increasing need for a greener route for metal–metal oxide nanocomposite synthesis.
Electrochemically active microorganisms form electrochemically active biofilms (EABs) on solid surfaces and have potential applications in bioenergy and chemical production.19–21 EABs have attracted considerable attraction in bio electrochemical systems (BESs), such as microbial fuel cells, where they act as a living bioanode that produce an excess of electrons and protons by biologically oxidizing a range of substrates. The flow of these electrons produces a considerable amount of electricity. Recent studies have shown that the EABs can be used to synthesize noble metal nanoparticles, and metal–metal oxide nanocomposites.2,12,19
This paper reports the biogenic synthesis of Ag–SnO2 nanocomposites (Ag–SnO2) using an EAB. The as-prepared Ag–SnO2 was used as a highly visible-light-driven photocatalyst towards the degradation of methyl orange (MO), methylene blue (MB), 4-nitrophenol (4-NP) and 2-chlorophenol (2-CP). Photoelectrochemical measurements, such as electrochemical impedance spectroscopy (EIS), linear scan voltammetry (LSV) and differential pulse voltammetry (DPV) in the dark and under visible light irradiation, further supported the visible light activity of the Ag–SnO2 nanocomposites. The photodegradation efficiency and photoelectrochemical responses of the Ag–SnO2 nanocomposites were significantly higher than those of pure SnO2 (p-SnO2). This study suggests that Ag–SnO2 nanocomposites are promising candidates for photocatalysis and photoelectrode materials.
Experimental
Materials
Silver nitrate (AgNO3, 99% pure), tin oxide nanoparticles (SnO2), MB and 2-CP were purchased from Sigma-Aldrich. Sodium acetate, MO, 4-NP and sodium sulfate (Na2SO4) were obtained from Duksan Pure Chemicals Co. Ltd. South Korea and used as received. α-Terpineol and ethyl cellulose were purchased from KANTO Chemical Co., Japan and fluorine-doped transparent conducting oxide glass (FTO; F-doped SnO2 glass; 7 Ω sq−1) was purchased from Pilkington, USA. Carbon paper (without wet proof, Fuel Cell Earth LLC, USA), and all other chemicals used in this study were of analytical grade and used as received. All solutions were prepared in deionized water that was obtained using a PURE ROUP 30 water purification system.Methods
X-ray diffraction (XRD, PANalytical, X'pert PRO-MPD, Netherland) was carried out using Cu Kα radiation (λ = 0.15405 nm). The XRD peaks of the crystalline phases were compared with those of standard compounds reported in the JCPDS data file. A UV-VIS-NIR spectrophotometer (Cary 5000, VARIAN, USA) was used to record the diffuse reflectance/absorbance spectra (DRS) of the p-SnO2 and Ag–SnO2 nanocomposites (1 mM and 3 mM Ag–SnO2) samples in the range, 200–800 nm. X-ray photoelectron spectroscopy (XPS, ESCALAB 250 XPS System, Thermo Fisher Scientific U.K.) was performed using a monochromatized Al Kα X-ray source (hν = 1486.6 eV) at Korea Basic Science Institute (KBSI). The binding energy of C 1s (284.60 eV) was used to calibrate the other binding energies. The size and distribution of the Ag–SnO2 and p-SnO2 nanoparticles were observed by field emission transmission electron microscopy (FE-TEM, Tecnai G2 F20, FEI, USA) with an accelerating voltage of 200 kV combined with energy dispersive spectrometry (EDS). The photoluminescence (PL, Kimon, 1 K, Japan) of the samples (p-SnO2 and Ag–SnO2) were recorded over the range, 200–800 nm at KBSI. The photoelectrochemical and photocatalytic experiments were performed using a 400 W lamp with an intensity of 31 mW cm−2 and λ > 400 nm (3M, USA). EIS and LSV were performed in a three electrode cell with a 0.2 M Na2SO4 aqueous solution as the electrolyte using a potentiostat (VersaSTAT 3, Princeton Research, USA). The DPV of the p-SnO2 and Ag–SnO2 nanocomposites photoelectrodes were recorded with a pulse height of 50 mV, pulse width of 0.005 s and scan rate of 4 mV s−1. The working electrodes were prepared as follows. 100 mg of p-SnO2 and Ag–SnO2 (1 mM and 3 mM) were suspended thoroughly using a conditioning mixer by adding ethyl cellulose as a binder and α-terpineol as a solvent for the paste. The resulting mixture was then coated on a FTO glass electrode using the doctor-blade method. The p-SnO2 and Ag–SnO2-coated (FTO) glass substrates were used as the working electrode. Ag/AgCl (saturated with KCl) and a Pt gauge were used as the reference and counter electrodes.Electrochemically active biofilm preparation
The EABs were developed on plain carbon paper according to previous studies.19–21 In a typical procedure, carbon paper with a size of 2.5 cm × 4.5 cm was dipped into a mineral salt medium containing sodium acetate (1 g L−1) as a substrate in a 250 mL bottle. 10 mL of anaerobic sludge (from a biogas plant in Paju, Korea) was added under strict anaerobic conditions by sparging N2 gas for 5 min to remove environmental oxygen contamination. All media, including the bacterial inoculum, were changed every two days under strict anaerobic conditions. This process was repeated for two weeks, and a living EAB was formed on the surface of the carbon paper.Synthesis of Ag–SnO2 nanocomposites
The EABs developed on carbon paper were used to synthesize the Ag–SnO2 nanocomposite, as shown in Fig. 1. In a typical process, aqueous solutions of AgNO3 (1 mM and 3 mM) were added to a 200 mL suspension of SnO2 nanoparticles (4 mM). Subsequently, 0.2 g sodium acetate (1 g L−1) was added as an electron donor. The mixture of AgNO3 and SnO2 nanoparticles was stirred for 5 min to allow the complete adsorption of Ag+ ions on the p-SnO2 surface. After the complete adsorption of Ag+ ions on the p-SnO2 surface, EAB was hung in a bottle. EAB produced electrons under anaerobic conditions, resulting in the reduction of Ag+ ions on the p-SnO2 surface. The color change after 12 h indicated the formation of Ag–SnO2 in the solution, which was centrifuged to isolate the Ag–SnO2. The isolated Ag–SnO2 was dried in an oven at 90 °C for 10 hours, and stored in a desiccator until required.Two control experiments were further performed to check the role of the EAB and sodium acetate. One was performed in the absence of an electron donor (sodium acetate), in which only the prepared EAB was hung in a SnO2 and AgNO3 solution under anaerobic conditions. The other controlled experiment was performed in the absence of the EAB by adding only an electron donor (sodium acetate) in a SnO2 and AgNO3 mixture under anaerobic conditions. In both the controlled experiments, no color change was observed, even after 24 h. These experiments confirmed the role of EAB and acetate in the synthesis of Ag–SnO2 nanocomposites.
Photocatalytic degradation
The photocatalytic activities of the Ag–SnO2 nanocomposites were evaluated by the decomposition of MO, MB, 4-NP and 2-CP under visible light irradiation. 2 mg of each (p-SnO2 and Ag–SnO2) photocatalyst was dispersed in an aqueous solution in two different 20 mL MO (10 mg L−1), 20 mL MB (10 mg L−1), 20 mL 4-NP (5 mg L−1) and 20 mL 2-CP (50 mg L−1) solutions, and agitated for 10 min in the dark to achieve adsorption–desorption equilibrium. The above suspensions were irradiated with visible light and after 1 h, a 2 mL sample of the solution was taken and the catalyst was separated from the solution by centrifugation to obtain a clear liquid. The concentrations of MO, MB, 4-NP and 2-CP were analyzed by measuring the absorbance at the characteristic wavelengths using a UV-vis spectrophotometer. The reaction kinetics and decolorization efficiency of the photocatalyst were determined using the method reported elsewhere.2,6,12Photoelectrochemical studies (EIS, LSV and DPV)
To examine the photoelectrochemical response of p-SnO2 and Ag–SnO2, DPV, EIS and LSV experiments were carried out under ambient conditions in the dark and under visible light irradiation. The DPV experiment was carried out under ambient conditions in 50 mL of a 0.2 M phosphate buffer solution (pH = 7), whereas the EIS and LSV experiments were performed in 50 mL of an aqueous 0.2 M Na2SO4 solution at room temperature. For each electrode, EIS was first performed in the dark and later under visible light irradiation (λ > 400 nm) at 0.0 V and with a frequency ranging from 1 to 104 Hz. The photocurrent response was examined by LSV in the dark and under visible light irradiation at a scan rate of 50 mV s−1 over a potential range, −0.9 to 1.0 V.Results and discussion
Proposed reaction mechanism for the synthesis of the Ag–SnO2 nanocomposites
The EAB was used as an innovative and green tool to synthesize the Ag–SnO2 nanocomposites (1 mM and 3 mM), employing a low-cost, surfactant-free and environmentally-friendly method. The EABs provide an excess of electrons and protons by biologically decomposing sodium acetate.19–21 These electrons reduce Ag+ ions to Ag0 at the surface of the SnO2 nanostructure (Fig. 1). The advantage of this synthesis is that it does not involve external energy input and normally occurs in water at room temperature. This makes the synthesis highly useful and efficient for nanocomposite synthesis.2,6,12,19,20X-ray diffraction analysis
Fig. 2 presents the XRD patterns of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites. Two sets of XRD patterns were obtained, those unmarked were indexed to the crystalline tetragonal structure of SnO2 (JCPDS no. 41-1445),6 whereas the other peaks marked with “*” were assigned to face centered cubic (fcc) Ag (JCPDS no. 04-0783).2,12,13,17 The absence of any other XRD peaks indicates the high purity of the sample. In addition, the crystallize size calculated using the Scherrer’s formula, was ∼11.00 nm, ∼14.20 and 14.57 nm for the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites, respectively.2,12,13 A significant change in the crystallite size of the Ag–SnO2 nanocomposites was observed, showing that the small AgNPs had deposited on the surface of the p-SnO2 nanoparticles.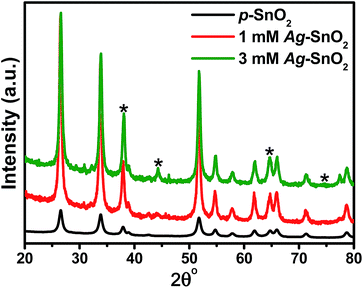 | ||
| Fig. 2 XRD patterns of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites. The peaks marked with (*) were assigned to AgNPs. | ||
Optical properties
The light absorption characteristics of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites were examined by UV-visible diffuse reflectance (Fig. S1†) and absorbance spectroscopy (Fig. 3a). The spectrum for p-SnO2 shows that they absorb mainly in the UV range because of their wide band gap. Moreover, the 1 mM and 3 mM Ag–SnO2 nanocomposites showed a broad absorption peak from 400–550 nm, which can be attributed to the surface plasmon resonance absorption of AgNPs.2,13 This further confirms that AgNPs had been deposited successfully on the p-SnO2 surface. Interestingly, Ag–SnO2 nanocomposites prepared from the silver precursor at different concentrations (1 mM and 3 mM AgNO3) exhibited different and higher absorption intensity in the visible region compared to p-SnO2. The extended absorbance of the Ag–SnO2 nanocomposites in the visible region is expected to improve the photocatalytic activity under sunlight or visible light irradiation. This shift in the absorption spectra of the Ag–SnO2 nanocomposites also suggests an interaction between Ag and p-SnO2, which is also in accordance with the XRD patterns.2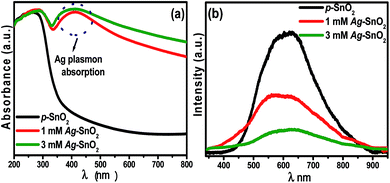 | ||
| Fig. 3 (a) UV-vis diffuse absorption spectra, and (b) photoluminescence spectra of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites. | ||
Fig. 3b shows the PL spectra of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites. The intensity of the PL spectrum is related directly to the electron–hole recombination rate, i.e. more the intense the spectrum, the higher the rate of electron–hole recombination.22 Alternatively, a lower intensity indicates that more excited electrons are trapped and transferred stably through the interface.2,12,13,22 p-SnO2 exhibits the strongest emission intensity of the PL spectrum, confirming the fastest charge recombination rate, whereas in the case of the 1 mM and 3 mM Ag–SnO2 nanocomposites, the PL intensity was reduced significantly after the anchoring of AgNPs because Ag can trap photo-generated electrons to facilitate charge separation.2,12,13,22 In general, the efficient charge separation and the inhibited electron–hole recombination by AgNPs are favorable for enhancing the photocatalytic activity of p-SnO2. The PL spectra showed that anchoring the AgNPs to the surface of p-SnO2 can effectively inhibit the electron–hole recombination during a photocatalytic reaction under visible light irradiation.
TEM analysis
The microstructural characteristics of the p-SnO2 and Ag–SnO2 nanocomposites were observed by TEM and HR-TEM. Fig. S2 and S3† shows TEM and HR-TEM images of the p-SnO2 nanoparticles, respectively. Fig. 4 shows the TEM and HR-TEM images of the Ag–SnO2 nanocomposites. As shown in Fig. 4b and b′, AgNPs with a particle size of ∼5–10 nm were attached to the surface of the p-SnO2 nanoparticles. From the HR-TEM image of the Ag–SnO2 nanocomposites, the AgNPs and SnO2 could be identified clearly by the lattice fringes (Fig. 4c and c′). The lattice fringes with d = 0.26 nm spacing were assigned to the Ag (111) planes and the lattice fringes with d = 0.33 nm were assigned to the (110) plane of SnO2. Furthermore, Fig. 4c and c′ clearly shows that the AgNPs were attached to the surface of the SnO2 nanoparticles. The SAED pattern in Fig. S4–S6† also confirmed the single crystalline nature of the materials. Fig. S7–S9† show HAADF-STEM images of the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites, respectively. Fig. S10–S12† shows the EDX spectra of the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites, respectively, corresponding to the Sn (K), O (K) and Ag (K) lines. EDX (Fig. S11 and S12†) further confirmed the presence of Sn, Ag and O in the samples. These results are in good agreement with the XRD patterns, and show that the Ag–SnO2 nanocomposites has a highly crystalline structure with a small and relatively uniform distribution of AgNPs at the SnO2 surface.XPS analysis
XPS was used to examine the chemical states and surface composition of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites. The XP survey spectra of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites (Fig. 5a) revealed three major sets of peaks for the Sn 3d peaks, O 1s peaks and Ag 3d peak, which exist in the samples and no trace of any impurity was observed. The carbon peak in Fig. S13† (C 1s = 284.8 eV) was assigned to the residual carbon from the sample and hydrocarbons from the XPS instrument. The high-resolution XP spectra of Sn 3d, O 1s and Ag 3d (Fig. 5b–d) showed slightly different binding energies of these electrons in the case of SnO2, suggesting an interaction between the Ag and SnO2 nanoparticles. Furthermore, two XPS peaks of the Sn 3d5/2 and Sn 3d3/2 core level states of Sn centered at 486.8 and 495.4 eV corresponds to the binding energy of Sn4+ in SnO2.6 In Fig. 5c, the O 1s spectra of the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites showed a single symmetrical peak at ∼530.46 ± 0.05 eV, corresponding to the lattice oxygen of SnO2 nanoparticles.6 | ||
| Fig. 5 XP spectra of p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites (a) survey spectra, (b) Sn 3d peaks, (c) O 1s peaks, and (d) Ag 3d peak. | ||
Fig. 5d shows the photoelectron peak of Ag 3d. The Ag 3d photoelectron peaks showed two individual peaks at 367.5 ± 0.02 eV for Ag 3d5/2 and 373.5 ± 0.02 eV for Ag 3d3/2.2,4,13,23 The 6.0 eV difference between the binding energy of these photoelectron peaks is also characteristic of metallic Ag, which is further evidence of the reduction of Ag+ ions by the EAB.
Visible light photocatalytic performance
The photocatalytic activity of the as-prepared Ag–SnO2 nanocomposites (1 mM and 3 mM) were evaluated by the degradation of colored dyes (MO and MB) and non-colored organic compounds (4-NP and 2-CP) as model reaction under visible light irradiation. Fig. 6a–d shows the photocatalytic degradation kinetics of MO, MB, 4-NP and 2-CP, whereas Fig. S14a–S14d† show the degradation (C/C0) of MO, MB, 4-NP and 2-CP as a function of the irradiation time. Here, C is the absorption of MO, MB, 4-NP or 2-CP solution at each time interval of irradiation, and C0 is the absorption of the initial concentration (time 0). After visible light irradiation on MO and MB for 6 h and 5 h, respectively, almost complete degradation occurred. The change in the concentration of MO and MB as a function of the reaction time for the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites exhibited pseudo-first-order kinetics according to the equation reported elsewhere.6 The rate constants (k) of the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites for the degradation of MO were 0.02408/h (R2 = 0.9956), 0.0894/h (R2 = 0.9970) and 0.2378/h (R2 = 0.9879), respectively. Similarly, the rate constants of the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites for the degradation of MB were 0.05578/h (R2 = 0.9884), 0.1888/h (R2 = 0.9987) and 0.2897/h (R2 = 0.9974), respectively. The k value for the visible light degradation of MO and MB by the 3 mM Ag–SnO2 nanocomposites was ∼10 and 6 times higher, respectively, than that of p-SnO2. The Ag–SnO2 nanocomposites showed enhanced visible light photocatalytic activity compared to p-SnO2 and other reported metal–metal oxide nanocomposites.2,12,13 | ||
| Fig. 6 ln(C/C0) versus time (h) plot for the photodegradation of (a) MO, (b) MB, (c) 4-NP, and (d) 2-CP by p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites under visible light irradiation. | ||
Moreover, the sensitization effects of dyes were not excluded because of the absorbance of dyes in the visible region. To further verify this effect, the photocatalytic degradation of non-colored phenolic compound such as 4-NP and 2-CP, which did not absorb the light in the visible region, was also performed under visible light irradiation (Fig. 6c and d, S14c and d†).24,25 The k value of the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites for the degradation of 4-NP were 0.0207/h (R2 = 0.9970), 0.0545/h (R2 = 0.9850) and 0.1157/h (R2 = 0.9973), respectively. Similarly, the rate constants of the p-SnO2, 1 mM and 3 mM Ag–SnO2 nanocomposites for the degradation of 2-CP were 0.0286/h (R2 = 0.9970), 0.0638/h (R2 = 0.9970) and 0.1116/h (R2 = 0.9997), respectively. The k value for the visible light degradation of 4-NP and 2-CP by the 3 mM Ag–SnO2 nanocomposites was ∼6 and 4 times higher, respectively, than that of p-SnO2. The results indicated that the photocatalytic activity of p-SnO2 was significantly improved by anchoring with AgNPs. The higher observed photocatalytic activity of Ag–SnO2 compared to p-SnO2 suggests that the AgNPs anchored at the surface of p-SnO2 and behave as an electron sink, which increases the separation of the photogenerated electron–hole pairs significantly and inhibits their recombination. This suggests that the AgNPs at the surface of p-SnO2 enhanced the photocatalytic activity of the p-SnO2 for the effective degradation of dyes and other organic pollutants under visible light irradiation.
Mechanism for degradation of the MO, MB, 4-NP and 2-CP
Fig. 7 presents the mechanistic profile of the photoinduced charge separation, migration and degradation process under visible light irradiation. Metal–metal oxide nanocomposites,26 such as Au–CeO212 and Ag–ZnO,2,13 can be used as highly-active photocatalysts because of the synergistic effect between the metal oxide and the surface plasmon resonance effect of the noble metal. Generally, a Schottky barrier is formed when two materials with different work functions are combined, and electrons are transferred from the materials with the lower work function to the materials with the higher work function until the two levels reach equilibrium to form a new Fermi energy level.2,12,13,26 The equilibrium alignment of the Fermi level of the metal and metal oxide nanocomposite materials creates a built-in electric field in the space charge region near the interface, which promotes the separation of photogenerated electrons and holes, and enhances the photocatalytic activity.2,12,17,26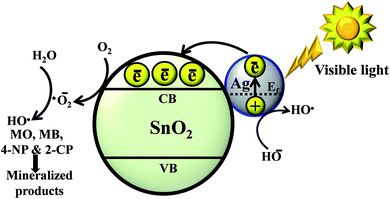 | ||
| Fig. 7 Schematic diagram of the photocatalytic mechanism for the degradation of MO, MB, 4-NP and 2-CP in the presence of Ag–SnO2 nanocomposites under visible light irradiation. | ||
When subjected to visible-light irradiation, the Ag–SnO2 nanocomposites were excited due to the surface plasmon resonance of the AgNPs, resulting in the generation of electron–hole pairs on the surface of the AgNPs. The photoexcited electrons transfer rapidly occurs from Ag to SnO2 through the interface between the AgNPs and SnO2.2,9,12,13,26,27 These injected electrons are trapped by dissolved oxygen molecules in water to yield a high oxidative species, such as super-oxide radical anions (˙O2−) and hydroxyl radicals (HO˙).2,8,12 On the other hand, some of the photoinduced holes on the surface of the AgNPs can be trapped by OH− to produce HO˙ species, which is an extremely strong oxidant for the partial or complete mineralization of organic chemicals. The trapping nature of the AgNPs also results in the production of more super oxide radical anions. Super-oxide radical anions (˙O2−) and hydroxyl radicals (HO˙) produced under visible light irradiation might induce the mineralization of organic pollutants.2,8,12,28 In general, the photocatalytic activity has a positive correlation with the rate of reactive radical formation, i.e. faster forming radicals leads to a higher photocatalytic activity of the catalyst. Overall, these results suggest that the AgNPs at the surface of the SnO2 nanoparticles will help increase the rate of formation of ˙O2− and HO˙ reactive radicals, facilitating the degradation of organic pollutants.
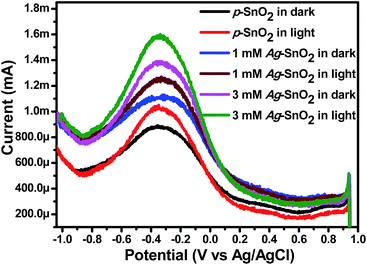
Photoelectrochemical studies
The enhanced performance of the visible light photocatalytic reaction for the degradation of MO, MB, 4-NP and 2-CP, and the photoelectrochemical studies (EIS, LSV and DPV) shown by Ag–SnO2 nanocomposites confirmed that the AgNPs had been deposited on the SnO2 surface. The interfacial interaction and charge transfer between the AgNPs and SnO2 could be responsible for the enhanced visible light activities of the Ag–SnO2 nanocomposites.
Stability and reusability of the Ag–SnO2 nanocomposites
The stability of nanocomposites is a major issue of concern. This study examined the stability of the nanocomposite by sonicating a suspension of the Ag–SnO2 nanocomposites in water for one hour. The centrifuged solution was then analyzed for the leached AgNPs using a UV-visible spectrophotometer (Fig. S15†). The AgNPs showed no absorbance peak (Fig. S15†). The reusability of Ag–SnO2 was tested by centrifuging the catalyst from the dye solution, followed by washing with DI water and drying in an air oven at 100 °C. The reused catalyst showed a similar response (Fig. S16a and S16b†) to that of the fresh catalyst, highlighting the stability and reusability of the Ag–SnO2 nanocomposites.Conclusions
Ag–SnO2 nanocomposites with different concentrations of silver precursor (1 mM and 3 mM) were synthesized using an EAB without toxic chemicals, surfactants and organic solvents. Compared to pure SnO2 the Ag–SnO2 nanocomposites showed enhanced photocatalytic performance for the photodecomposition of MO, MB, 4-NP and 2-CP under visible light irradiation. The enhanced photocatalytic activities of Ag–SnO2 were attributed mainly to the introduction of AgNPs, which facilitated charge separation of the photogenerated electrons and holes. The anchoring of AgNPs on the p-SnO2 surface improves the separation and transfer of charge carriers under visible light irradiation, through which the visible light photoactivity of the Ag–SnO2 nanocomposites were enhanced.The photoelectrochemical (EIS, LSV and DPV) response highlighted the enhanced photoactivity of the Ag–SnO2 nanocomposites under visible light irradiation. This study establishes a simple and green method for preparing highly visible light-active Ag–SnO2 nanocomposite materials for possible applications in industrial effluent treatment and photoelectrode materials. This environmentally friendly synthesis is expected to provide a new means of synthesizing a series of metal–metal oxide photocatalysts for use in photo-assisted catalytic reactions.
Acknowledgements
This study was supported by the 2013 Yeungnam University Research Grant, South Korea.Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS
.
- S. A. Ansari, M. M. Khan, M. Omaish, J. Lee and M. H. Cho, J. Phys. Chem. C, 2013, 117, 27023–27030 CAS
.
- S. H. S. Chan, T. Y. Wu, J. C. Juan and C. Y. Teh, J. Chem. Technol. Biotechnol., 2011, 86, 1130–1158 CrossRef CAS
.
- H. Chen, C. E. Nanayakkara and V. H. Grassian, Chem. Rev., 2012, 112, 5919–5948 CrossRef CAS PubMed
.
- M. M. Hassan, W. Khan, A. Azam and A. H. Naqvi, J. Lumin., 2014, 145, 160–166 CrossRef CAS PubMed
.
- S. A. Ansari, M. M. Khan, M. Omaish, J. Lee and M. H. Cho, New J. Chem., 2014, 38, 2462–2469 RSC
.
- Z. Zhao, H. Tan, H. Zhao, Y. Lv, L. Zhou and Y. Song, Chem. Commun., 2014, 50, 2755–2757 RSC
.
- R. Saravanan, N. Karthikeyan, V. K. Gupta, E. Thirumal, P. Thangadurai and V. Narayanan, Mater. Sci. Eng., C, 2013, 33, 2235–2244 CrossRef CAS PubMed
.
- P. Wang, B. Huang, Y. Dai and M. H. Whangbo, Phys. Chem. Chem. Phys., 2012, 14, 9813–9825 RSC
.
- L. Jing, W. Zhou, G. Tian and H. Fu, Chem. Soc. Rev., 2013, 42, 9509–9549 RSC
.
- S. T. Kochuveedu, Y. H. Jang and D. H. Kim, Chem. Soc. Rev., 2013, 42, 8467–8493 RSC
.
- M. M. Khan, S. A. Ansari, M. O. Ansari, B. K. Min, J. Lee and M. H. Cho, J. Phys. Chem. C, 2014, 118, 9477–9484 CAS
.
- S. A. Ansari, M. M. Khan, J. Lee and M. H. Cho, J. Ind. Eng. Chem., 2014, 20, 1602–1607 CrossRef CAS PubMed
.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014 10.1039/C3CS60425J
.
- Z. Khan, T. R. Chetia, A. K. Vardhaman, D. Barpuzary, C. V. Sastri and M. Qureshi, RSC Adv., 2012, 2, 12122–12128 RSC
.
- C. M. Fan, Y. Peng, Q. Zhu, L. Lin, R. X. Wang and A. W. Xu, J. Phys. Chem. C, 2013, 117, 24157–24166 CAS
.
- W. Wu, L. Liao, S. Zhang, J. Zhou, X. Xiao, F. Ren, L. Sun, Z. Dai and C. Jiang, Nanoscale, 2013, 5, 5628–5636 RSC
.
- H. J. You, R. Liu, C. C. Liang, S. C. Yang, F. Wang, X. G. Lu and B. J. Ding, J. Mater. Chem. A, 2013, 1, 4097–4104 CAS
.
- S. Kalathil, M. M. Khan, J. Lee and M. H. Cho, Biotechnol. Adv., 2013, 31, 915–924 CrossRef CAS PubMed
.
- M. M. Khan, S. A. Ansari, J. H. Lee, J. Lee and M. H. Cho, ACS Sustainable Chem. Eng., 2014, 2, 423–432 CrossRef CAS
.
- T. H. Han, M. M. Khan, S. Kalathil, J. Lee and M. H. Cho, Ind. Eng. Chem. Res., 2013, 52, 8174–8181 CrossRef CAS
.
- J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang and S. Jiazhong, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef PubMed
.
- C. Chen, Y. Zheng, Y. Zhan, X. Lin, Q. Zheng and K. Wei, Dalton Trans., 2011, 40, 9566–9570 RSC
.
- G. Wang, X. Wang, J. Liu and X. Sun, Chem.–Eur. J., 2012, 18, 5361–5366 CrossRef CAS PubMed
.
- N. N. Rao, A. K. Dubey, S. Mohanty, P. Khare, R. Jain and S. N. Kaul, J. Hazard. Mater., 2003, 101, 301–314 CrossRef CAS
.
- C. Clavero, Nat. Photonics, 2014, 8, 95–103 CrossRef CAS
.
- Z. Chen, L. Fang, W. Dong, F. Zheng, M. Shen and J. Wang, J. Mater. Chem. A, 2014, 2, 824–832 CAS
.
- M. Qamar and A. Khan, RSC Adv., 2014, 4, 9542–9550 RSC
.
- R. W. Murray, Chem. Rev., 2008, 108, 2688–2720 CrossRef CAS PubMed
.
- S. Kalathil, J. Lee and M. H. Cho, J. Nanopart. Res., 2013, 14, 1051–1060 CrossRef
.
- M. M. Khan, S. A. Ansari, J. Lee and M. H. Cho, J. Ind. Eng. Chem., 2013, 19, 1845–1850 CrossRef CAS PubMed
.
- X. Bai, L. Wang, R. Zong, Y. Lv, Y. Sun and Y. Zhu, Langmuir, 2013, 29, 3097–3105 CrossRef CAS PubMed
.
- L. Zhao, L. Fang, W. Dong, F. G. Zheng and M. R. Shen, Appl. Phys. Lett., 2013, 102, 121905 CrossRef PubMed
.
- Y. Lv, Y. Zhu and Y. Zhu, J. Phys. Chem. C, 2013, 117, 18520–18528 CAS
.
- W. H. Leng, Z. Zhang, J. Q. Zhang and C. N. Cao, J. Phys. Chem. B, 2005, 109, 15008–15023 CrossRef CAS PubMed
.
- J. Gan, X. Lu, J. Wu, S. Xie, T. Zhai, M. Yu, Z. Zhang, Y. Mao, S. C. Wang, Y. Shen and Y. Tong, Sci. Rep., 2013, 3, 1021–1028 Search PubMed
.
- S. A. Ansari, M. M. Khan, M. O. Ansari, S. Kalathil, J. Lee and M. H. Cho, RSC Adv., 2014, 4, 16782–16791 RSC
.
- M. M. Khan, S. A. Ansari, D. Pradhan, M. Omaish, D. H. Han, J. Lee and M. H. Cho, J. Mater. Chem. A, 2014, 2, 637–644 CAS
.
- H. M. Chen, C. K. Chen, C. J. Chen, L. C. Cheng, P. C. Wu, B. H. Cheng, Y. Z. Ho, M. L. Tseng, Y. Y. Hsu and T. S. Chan, ACS Nano, 2012, 6, 7362–7372 CrossRef CAS PubMed
.
- M. O. Ansari, M. M. Khan, S. A. Ansari, K. Raju, J. Lee and M. H. Cho, ACS Appl. Mater. Interfaces, 2014 DOI:10.1021/AM500488E
.
- M. M. Khan, S. A. Ansari, D. Pradhan, D. H. Han, J. Lee and M. H. Cho, Ind. Eng. Chem. Res., 2014 DOI:10.1021/IE500986N
.
Footnote |
| † Electronic supplementary information (ESI) available: DRS spectra of p-SnO2 and Ag–SnO2, TEM and HRTEM images of the p-SnO2, SAED, HAADF and EDX of p-SnO2 and Ag–SnO2, C 1s spectra of p-SnO2 and Ag–SnO2, photodegradation spectra of MO, MB, 4-NP and 2-CP, UV-Vis spectra of Ag–SnO2 for Ag leaching and recycled photodegradation plots of MO and MB. See DOI: 10.1039/c4ra03448a |
| This journal is © The Royal Society of Chemistry 2014 |