Current research and future perspectives of phytase bioprocessing
Abstract
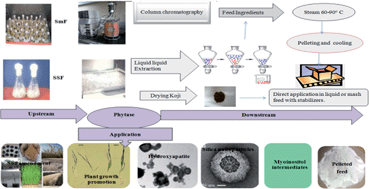
Phosphorus is one of nature's paradoxes as it is life's bottleneck for subsistence on earth but at same time is detrimental in surplus quantities in an aquatic environment. Phosphorus cannot be manufactured, though fortunately it can be recovered and reused. The only way to avert a supply crisis is to implement the “3R's” of sustainability, “Reduce, Reuse and Recycle.” Phytase is likely to play a critical role in the dephosphorylation of antinutritional and indigestible phytate, a phosphorus locking molecule, to digestible phosphorus, calcium and other mineral nutrients in the coming years. Hence, efforts are required to produce cost effective phytase with fast upstream and economic downstream processing because the current available process is expensive and time consuming. This review summarizes the present state of methods studied for phytase bioprocessing. Production, extraction and purification incur a large cost in product development. In addition, the process has several limitations such as dilute concentration of enzyme, extensive downstream procedures and treatment of generated effluents. However, these methods are currently employed due to lack of alternative methods. Thus, there is a clear need for an efficient, scalable and economical process for phytase production and bioseparation, and improvements are especially required with regard to yield, purity, and energy consumption. Perspectives for an improved bioprocess development for phytase are discussed based on our own experience and recent studies. It is argued that the optimization of production techniques, strain improvement and liquid–liquid extraction deserves more attention in the future.

 Please wait while we load your content...
Please wait while we load your content...