Facile fabrication of CuO nanowire modified Cu electrode for non-enzymatic glucose detection with enhanced sensitivity†
Pengjuan Niab,
Yujing Suna,
Yan Shiab,
Haichao Daiab,
Jingting Huab,
Yilin Wangab and
Zhuang Li*a
aState Key Laboratory of Electroanalytical Chemistry, Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin 130022, P. R. China. E-mail: zli@ciac.ac.cn; Fax: +86 431 85262057; Tel: +86 431 85262057
bUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 17th June 2014
Abstract
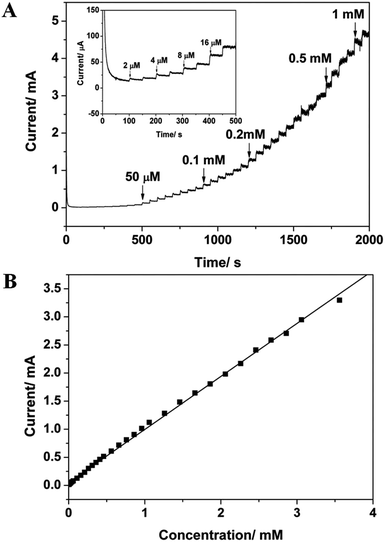
In this paper the fabrication of CuO nanowires (CuO NWs) by a facile two-step method is reported. Cu(OH)2 nanowires (Cu(OH)2 NWs) on a copper surface were prepared at room temperature by a simple solution-based procedure, and subsequent calcinations of Cu(OH)2 NWs led to the formation of CuO NWs. The morphologies and structures of Cu(OH)2 NWs and CuO NWs were characterized by scanning electron microscopy and X-ray diffraction. Electrochemical measurements showed that the CuO NWs modified Cu electrode exhibited good electrocatalytic behavior for the detection of glucose with a wide linear range from 2 μM to 3.56 mM (R2 = 0.9984), a low detection limit down to 0.05 μM, and a high sensitivity of 1886.3 μA mM−1 cm−2. The sensor also displayed a high selectivity, an acceptable reproducibility, an excellent long-term stability and good repeatability. Moreover, the as-prepared sensor has great potential in practical applications.
1. Introduction
Diabetes has become one of the major health care problems. Glucose, an essential bioactive substance, is often used as a clinical indicator of diabetes.1 Therefore, the reliable, accurate, and rapid determination of glucose is of practical importance in biological and clinical analysis.2 So far, various techniques such as electrochemistry, fluorimetry, surface plasmon resonance and capillary zone electrophoresis have been developed for glucose determination.3–6 Compared to other analytical techniques, electrochemistry methods have attracted more attention due to their intrinsic simplicity, good selectivity, high sensitivity and easy operation.7–10 The widely employed glucose sensors are enzyme-based because of their high sensitivity and excellent selectivity to glucose detection.11–15 However, most enzyme-based sensors suffer from high costs, short lifetimes and stability problems, which limit their further use.16 To overcome these disadvantages, numerous efforts have been made to develop non-enzymatic glucose sensors. Noble metals (Pt, Au, Pd) and their alloys (Pt–Pd, Pt–Au, Au–Pd) with large specific surface, excellent conductivity and electrocatalytic activities have been widely used for non-enzymatic glucose detection.17–24 However, these noble metals-based sensors have displayed the drawbacks of high cost that limit their practical application.9 Accordingly, it is crucial to develop cost-effective, enzyme-free glucose sensors.CuO, an important p-type semiconductor with a narrow band gap of 1.2 eV, has shown its potential applications in various fields, such as electrochemical sensors, photoelectric chemical materials, gas sensing and lithium ion batteries.25–28 Among these applications, electrochemical sensors have been intensively investigated. Recently, a few attempts have been made to amperometrically detect glucose based on CuO nanomaterials.8,10,29,30 Wang prepared the CuO nanofibers by electrospinning technique and used it for non-enzymatic glucose determination.31 Cherevko fabricated a CuO electrode by hydrogen bubble evolution and investigated its application in non-enzymatic glucose detection.32 However, the complex and time-consuming fabrication process limit their application. Therefore, it's still greatly demanded to develop simpler processes to synthesize novel CuO nanostructures with superior catalytic property for fast, sensitive, and stable detection of glucose.
In this paper, we reported a facile way to directly grow CuO NWs on a copper surface. Firstly Cu(OH)2 NWs on the copper surface was fabricated through a simple, template-free, solution-based procedure.33 The facile transformation of Cu(OH)2 NWs to CuO NWs without obvious morphological change has been achieved by heat treatment. The whole process is simple without using complicated and expensive equipment. The obtained CuO NWs modified Cu electrode exhibits good electrocatalytic performance toward the electrooxidation of glucose. Moreover, the proposed sensor has great potential in the practical applications.
2. Experimental section
2.1 Materials and apparatus
Copper foil and malt dust were purchased from Sangon Biotech Co., Ltd (Shanghai, China). Glucose, sucrose, NaOH and (NH4)2S2O8 were obtained from Beijing Chemical Co. (Beijing, China). Ascorbic acid (AA), uric acid (UA) and dopamine (DA) were supplied by Sigma-Aldrich. All of these reagents were of analytical grade and used without further purification. The ultrapure water used throughout this work was produced by a Milli-Q system. The copper foils were used after ultrasound cleaning in 3 M HCl, ethanol and deionized water.Scanning electron microscopy (SEM) characterizations were carried out using a S-4800 FE-SEM scanning electron microscope equipped with an accelerating voltage of 20 kV. The X-ray diffraction (XRD) patterns were recorded with a Rigaku-D/max 2500 V X-ray diffractometer equipped with a Cu Kα radiation source (λ = 1.54178 Å). Electrochemical measurements were performed on a CHI660a electrochemical workstation in a conventional three-electrode cell containing 0.1 M NaOH at room temperature, using a platinum foil as the counter electrode, a saturate calomel electrode (SCE) as the reference electrode and Cu electrode, Cu(OH)2 NWs modified Cu electrode and CuO NWs modified Cu electrode as the working electrodes.
2.2 Preparation of Cu(OH)2 NWs and CuO NWs
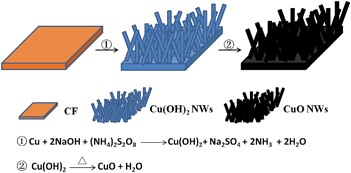
The formation mechanism of the CuO NWs is shown in Scheme 1. A typical fabrication of Cu(OH)2 NWs was carried out as follows. Briefly, a piece of clean copper foil (5 mm × 5 mm) was immersed into a solution containing 80 μL of 10 M NaOH, 180 μL H2O and 40 μL of 1 M (NH4)2S2O8. After immersing for 30 minutes, the copper foil was taken out from the solution, washed with distilled water and dried in the air. It can be seen clearly from Fig. S1† that a deep blue film is formed on the surface of copper foil. For the synthesis of CuO NWs, the Cu(OH)2 NWs modified Cu electrode was loaded into an alumina boat, which was then put in a tube furnace. After purging with Ar gas for 30 minutes, the furnace was heated to 120 °C for 3 h for complete dehydration. Then the temperature was raised to 180 °C and maintained at this temperature for 2 h to promote crystallization. As shown in Fig. S1,† the original blue color of the film turns into black after the heat treatment.3. Results and discussion
3.1 Characterization of copper foil, Cu(OH)2 NWs and CuO NWs
Fig. 1A and B show the SEM images of the cooper foil taken at different magnifications. It can be obviously seen that the surface of copper foil is coarse. The XRD peaks of the copper foil are displayed in Fig. 1C and the standard diffraction pattern for Cu (JCPDS no. 04-0836) is also presented for comparison. The peaks positions are in good agreement with the standard file, indicating the obtained copper foil has a high crystalline purity. The SEM images of Cu(OH)2 NWs are shown in Fig. 1D and E. It can be seen from the low-magnification SEM image (Fig. 1D) that the as-prepared Cu(OH)2 NWs shows a wire-like morphology and uniformly and compactly covers the copper foil. Fig. 1E reveals the wire-like structure of the Cu(OH)2 NWs with 50–500 nm in diameter. The XRD of Cu(OH)2 NWs (Fig. 1F) is attributed to the orthorhombic Cu(OH)2 phase. The peaks positions match well with the standard XRD data (JCPDS no. 13-0420), confirming the Cu(OH)2 NWs have been successfully grown on the copper foil. Fig. 1G and H display the low and high magnification SEM images of the CuO NWs. The wire-like morphology of the CuO NWs is well-preserved and the CuO NWs uniformly covers the copper foil surface. The XRD peaks of the CuO NWs are displayed in Fig. 1I. Except the small broad peak around 30 degrees from Cu(OH)2, no other peaks from impurities are observed compared with standard file (JCPDS no. 45-0937), demonstrating that the Cu(OH)2 NWs have been almost completely converted to CuO NWs after the heating treatment.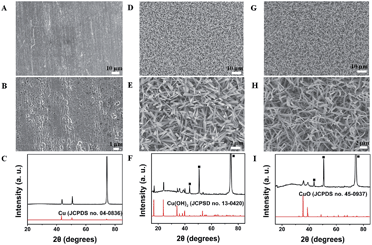 | ||
| Fig. 1 SEM images and XRD patterns of Cu electrode (A–C), Cu(OH)2 NWs (D–F), and CuO NWs (G–I). Peaks marked with ■ are from the copper foil. | ||
3.2 Electrocatalytic ability of CuO NWs modified Cu electrode towards glucose oxidation
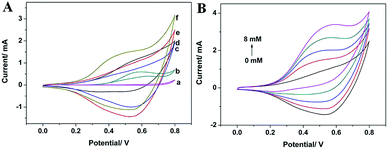
It was reported that CuO-based materials displayed excellent ability to catalyze the oxidation of glucose.34 In this study, the CuO NWs modified Cu electrode was fabricated and applied as an electrochemical sensor to investigate its catalytic performance on glucose oxidation with Cu electrode and Cu(OH)2 NWs modified Cu electrode as comparison. CV curves of different electrodes in 0.1 M NaOH in the potential window ranging from 0 to 0.8 V in the absence and presence of glucose are shown in Fig. 2A. In the absence of glucose, no obvious current signal is observed at Cu electrode (curve a), while a dramatic current signal is found at Cu(OH)2 NWs modified Cu electrode (curve c) and CuO NWs modified Cu electrode (curve e), respectively. After injecting 2 mM glucose in the electrolyte, a weak oxidation peak at ca. +0.58 V is observed for the Cu electrode (curve b) and a more significant oxidation signal of glucose at ca. +0.53 V is found at Cu(OH)2 NWs modified Cu electrode (curve d). For CuO NWs modified Cu electrode (curve f), a noticeable increase of the oxidation current starting at ca. +0.20 V with a shoulder peak at ca. +0.42 V is present. That may be attributed to the proposed involvement of Cu(II) and Cu(III) surface species in the oxidation of glucose though the exact mechanism for the oxidation of glucose in alkaline medium at the Cu-based modified electrode remains some-what controversial.25,35 The oxidation potential of glucose at CuO NWs modified Cu electrode is more negatively shifted than that at Cu(OH)2 NWs modified Cu electrode and Cu electrode. Furthermore, the oxidation current of glucose at CuO NWs modified Cu electrode is 1.5 times and 2.5 times than that at Cu(OH)2 NWs modified Cu electrode and Cu electrode, respectively. The more negative reduction potential and larger current response indicate that the CuO NWs modified Cu electrode has much higher catalytic activity towards the oxidation of glucose than Cu(OH)2 NWs modified Cu electrode and Cu electrode. As shown in Fig. 2B, a series of CVs are recorded in the absence and presence of glucose with different concentrations in the range of 2 mM to 8 mM. It can be clearly observed that the oxidation current increases with the increase of glucose concentration, indicating the CuO NWs modified Cu electrode has a good catalytic ability for the oxidation of glucose. To understand whether the dissolved oxygen involved in the electrocatalytic oxidation process, the amperometric responses of the CuO NWs modified Cu electrode to the successive injections of 0.2 mM glucose in 0.1 M NaOH with or without N2 degassing operation were investigated. Fig. S2† shows that the current responses to glucose remain the same magnitude when the solution is saturated with N2, suggesting the oxygen does not involve in this reaction.3.3 Amperometric performance of the CuO NWs modified Cu electrode to glucose
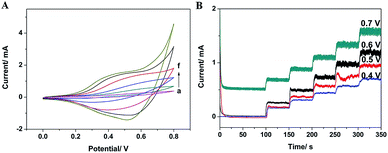
In order to ensure enough sensitivity and a lower background current, the effect of applied potential was also investigated by five successive amperometric measurements of 0.25 mM glucose into a stirring 0.1 M NaOH at different potentials ranging from 0.4 V to 0.7 V. As shown in Fig. 3B, the largest response sensitivity is obtained at 0.6 V. As a result, 0.6 V was applied as the working potential for the subsequent amperometric measurements.
| Type of electrode | Detection limit (μM) | Sensitivity (μA mM−1 cm−2) | Linear range (mM) | Reference |
|---|---|---|---|---|
| a Nanoleave-shaped copper oxide.b Multiple-walled carbon nanotube. | ||||
| CuO nanosphere | 1 | 404.53 | 0–2.55 | 8 |
| CuO flowers | 4 | 709.52 | 0.004–8 | 10 |
| CuO nanofibers | 0.8 | 431.3 | 0.006–2.5 | 31 |
| CuONLa/MWCNTsb | 5.7 | 664.3 | 0–0.9 | 36 |
| CuO nanourchins | 1.52 | 2682 | 0.1–3 | 37 |
| CuxO/Cu | 49.0 | 1620 | — | 38 |
| CuO nanobelt | <1 | 582.0 | 0.01–7.3 | 39 |
| CuO NLs | 5 | 26.6 μA mM−1 | 0.1–3 | 40 |
| CuO/TiO2 | 1 | 79.9 | 0–2.0 | 41 |
| CuO NWs | 0.05 | 1886.3 | 0.002–3.56 | This work |
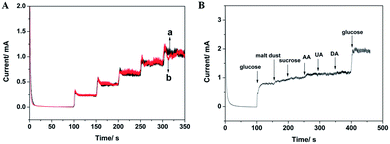
Previous reports have shown that glucose sensors based on Cu electrodes may easily lose their electrochemical activity due to the chloride poisoning effect.8,25 Therefore, the amperometric responses of CuO NWs modified Cu electrode to the successive injections of 0.2 mM glucose into 0.1 M NaOH with or without 0.2 M NaCl were investigated. As shown in Fig. 5A, the current responses of CuO NWs modified Cu electrode to glucose oxidation remain almost constant after adding NaCl into the electrolyte, indicating the electrode could be used in the presence of high concentration chloride ions.
The selectivity of the CuO NWs modified Cu electrode was also investigated because the co-existing electroactive species might affect the detection of glucose in real sample analysis. UA, DA and AA are the major interferences normally co-existing with glucose in real samples. Moreover, other carbohydrate compounds may also affect the determination of glucose. Considering that the normal physiological level of glucose is about 30 times than that of the interfering agents,42–44 the anti-interference effect of the as-prepared CuO NWs modified electrode was tested by successive addition of 1 mM glucose, and 0.2 mM of each of five relevant interfering species including sucrose, malt dust, AA, UA and DA. Fig. 5B shows that no obvious interference is observed, suggesting the sensor exhibits high selectivity to the detection of glucose.
4. Conclusions
In summary, we have successfully synthesized CuO NWs on a cooper surface by a simple two-step method. The CuO NWs were used to construct a non-enzymatic glucose sensor. The newly developed sensor displays good catalytic activity for glucose oxidation, with a wide linearity range, high sensitivity, good selectivity and low detection limit. In addition, the sensor also shows excellent stability and good selectivity to glucose detection. Moreover, the fabrication technique is simple and can be easily used for the mass production.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21275135), the National Basic Research Program of China (973 Program, no. 2010CB933600).Notes and references
- M. Liu, R. Liu and W. Chen, Biosens. Bioelectron., 2013, 45, 206 CrossRef CAS PubMed.
- S. K. Meher and G. R. Rao, Nanoscale, 2013, 5, 2089 RSC.
- S. Liu, J. Tian, L. Wang, X. Qin, Y. Zhang, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Catal. Sci. Technol., 2012, 2, 813 CAS.
- L. Jin, L. Shang, S. Guo, Y. Fang, D. Wen, L. Wang, J. Yin and S. Dong, Biosens. Bioelectron., 2011, 26, 1965 CrossRef CAS PubMed.
- W. Wu, J. Shen, Y. Li, H. Zhu, P. Banerjee and S. Zhou, Biomaterials, 2012, 33, 7115 CrossRef CAS PubMed.
- S. Wang, P. Su, E. Hongjun and Y. Yang, Anal. Biochem., 2010, 405, 230 CrossRef CAS PubMed.
- D. Nguyen Quoc, D. Patil, H. Jung and D. Kim, Biosens. Bioelectron., 2013, 42, 280 CrossRef PubMed.
- E. Reitz, W. Jia, M. Gentile, Y. Wang and Y. Lei, Electroanalysis, 2008, 20, 2482 CrossRef CAS.
- W. Lu, X. Qin, A. M. Asiri, A. O. Al-Youbi and X. Sun, Analyst, 2013, 138, 417 RSC.
- X. Wang, C. Hui, H. Liu, G. Du, X. He and Y. Xi, Sens. Actuators, B, 2010, 144, 220 CrossRef CAS PubMed.
- Z. Wang, S. Liu, P. Wu and C. Cai, Anal. Chem., 2009, 81, 1638 CrossRef CAS PubMed.
- J. Liu, N. Kong, A. Li, X. Luo, L. Cui, R. Wang and S. Feng, Analyst, 2013, 138, 2567 RSC.
- V. Mani, B. Devadas and S.-M. Chen, Biosens. Bioelectron., 2013, 41, 309 CrossRef CAS PubMed.
- B. Liang, L. Fang, G. Yang, Y. Hu, X. Guo and X. Ye, Biosens. Bioelectron., 2013, 43, 131 CrossRef CAS PubMed.
- T. Kong, Y. Chen, Y. Ye, K. Zhang, Z. Wang and X. Wang, Sens. Actuators, B, 2009, 138, 344 CrossRef CAS PubMed.
- Y. Zhang, X. Xiao, Y. Sun, Y. Shi, H. Dai, P. Ni, J. Hu, Z. Li, Y. Song and L. Wang, Electroanalysis, 2013, 25, 959 CrossRef CAS.
- L. Meng, J. Jin, G. Yang, T. Lu, H. Zhang and C. Cai, Anal. Chem., 2009, 81, 7271 CrossRef CAS PubMed.
- P. F. Luo, F. Z. Zhang and R. P. Baldwin, Anal. Chim. Acta, 1991, 244, 169 CrossRef CAS.
- F. Xiao, F. Zhao, D. Mei, Z. Mo and B. Zeng, Biosens. Bioelectron., 2009, 24, 3481 CrossRef CAS PubMed.
- Y. Zhang, F. Xu, Y. Sun, C. Guo, K. Cui, Y. Shi, Z. Wen and Z. Li, Chem.–Eur. J., 2010, 16, 9248 CrossRef CAS PubMed.
- X. Chen, H. Pan, H. Liu and M. Du, Electrochim. Acta, 2010, 56, 636 CrossRef CAS PubMed.
- A. Habrioux, E. Sibert, K. Servat, W. Vogel, K. B. Kokoh and N. Alonso-Vante, J. Phys. Chem. B, 2007, 111, 10329 CrossRef CAS PubMed.
- L. Rong, C. Yang, Q. Qian and X. Xia, Talanta, 2007, 72, 819 CrossRef CAS PubMed.
- X. Niu, M. Lan, C. Chen and H. Zhao, Talanta, 2012, 99, 1062 CrossRef CAS PubMed.
- Z. Zhuang, X. Su, H. Yuan, Q. Sun, D. Xiao and M. M. F. Choi, Analyst, 2008, 133, 126 RSC.
- C. Chiang, Y. Shin, K. Aroh and S. Ehrman, Int. J. Hydrogen Energy, 2012, 37, 8232 CrossRef CAS PubMed.
- G. Zhu, H. Xu, Y. Xiao, Y. Liu, A. Yuan and X. Shen, ACS Appl. Mater. Interfaces, 2012, 4, 744 CAS.
- B. Wang, X. Wu, C. Shu, Y. Guo and C. Wang, J. Mater. Chem., 2010, 20, 10661 RSC.
- X. Zhang, A. Gu, G. Wang, Y. Wei, W. Wang, H. Wu and B. Fang, CrystEngComm, 2010, 12, 1120 RSC.
- L. Jiang and W. Zhang, Biosens. Bioelectron., 2010, 25, 1402 CrossRef CAS PubMed.
- W. Wang, L. Zhang, S. Tong, X. Li and W. Song, Biosens. Bioelectron., 2009, 25, 708 CrossRef CAS PubMed.
- S. Cherevko and C. Chung, Talanta, 2010, 80, 1371 CrossRef CAS PubMed.
- W. X. Zhang, X. G. Wen, S. H. Yang, Y. Berta and Z. L. Wang, Adv. Mater., 2003, 15, 822 CrossRef CAS.
- G. Wang, Y. Wei, W. Zhang, X. Zhang, B. Fang and L. Wang, Microchim. Acta, 2010, 168, 87 CrossRef CAS.
- S. T. Farrell and C. B. Breslin, Electrochim. Acta, 2004, 49, 4497 CrossRef CAS PubMed.
- Z. Yang, J. Feng, J. Qiao, Y. Yan, Q. Yu and K. Sun, Anal. Methods, 2012, 4, 1924 RSC.
- S. Sun, X. Zhang, Y. Sun, S. Yang, X. Song and Z. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 4429 CAS.
- C. Li, Y. Su, S. Zhang, X. Lv, H. Xia and Y. Wang, Biosens. Bioelectron., 2010, 26, 903 CrossRef CAS PubMed.
- T. Soejima, H. Yagyu, N. Kimizuka and S. Ito, RSC Adv., 2011, 1, 187 RSC.
- Y. Zhao, J. Zhao, Y. Li, D. Ma, S. Hou, L. Li, X. Hao and Z. Wang, Nanotechnology, 2011, 22, 115604 CrossRef PubMed.
- S. Luo, F. Su, C. Liu, J. Li, R. Liu, Y. Xiao, Y. Li, X. Liu and Q. Cai, Talanta, 2011, 86, 157 CrossRef CAS PubMed.
- C. Kong, L. Tang, X. Zhang, S. Sun, S. Yang, X. Song and Z. Yang, J. Mater. Chem., 2014, 2, 7306 RSC.
- X. Zhang, S. Sun, J. Lv, L. Tang, C. Kong, X. Song and Z. Yang, J. Mater. Chem. A, 2014, 2, 10073 CAS.
- L. Jiang and W. Zhang, Biosens., 2010, 25, 1402 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03437f |
| This journal is © The Royal Society of Chemistry 2014 |