Bamboo-derived porous bioadsorbents and their adsorption of Cd(ii) from mixed aqueous solutions
Abstract
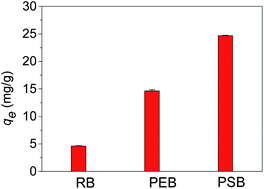
Two porous bioadsorbents were successfully prepared from the partial enzymatic hydrolysis of bamboo and subsequent chemical modification with succinic anhydride. We investigated the abilities of the partially enzymatically hydrolyzed bamboo (PEB) and the porous succinylated bamboo (PSB) to adsorb Cd(II) from a solution containing sodium chloride and an amino acid. The partial enzymatic hydrolysis created many pores within the raw bamboo, and both the porous structure and the post-succinylation carboxyl group content were important for high adsorption capacity. The presence of sodium chloride and the amino acid markedly decreased the adsorption capacities of the bioadsorbents. The experimental data could be described perfectly with the Langmuir adsorption isotherm model and pseudo second-order kinetics model. Even in solutions containing sodium chloride and arginine, the maximum Cd(II) adsorption capacities at 303 K were 38.18 and 120.34 mg g−1 for PEB and PSB, respectively.

 Please wait while we load your content...
Please wait while we load your content...