The decomposition of methanol on Au–Pt bimetallic clusters supported by a thin film of Al2O3/NiAl(100)
Abstract
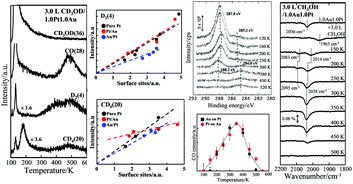
With various techniques to probe a surface, we studied the decomposition of methanol on Au–Pt bimetallic clusters, of diameter ≤6.0 nm, formed by sequential deposition of Au and Pt evaporated onto thin-film Al2O3/NiAl(100). The surface of the bimetallic clusters comprised both Au and Pt, but the decomposition, through dehydrogenation to CO and scission of the C–O bond, proceeded primarily on the surface Pt. Alloying of Pt with Au altered little the dehydrogenation on the Pt sites. The CO and hydrogen produced from dehydrogenated methanol increased with the extent of Pt sites; the production per surface Pt was comparable to that of Pt clusters. The temperature of the onset of dehydrogenation resembled that of Pt clusters. Little methanol decomposed to CO on the Au sites. Varying the surface structure and composition of the bimetallic clusters affected these properties insignificantly. In contrast to the dehydrogenation, scission of the C–O bond in methanol did not depend exclusively on the concentration of Pt atoms at the surface, given that production of methane from this second channel did not increase with the extent of Pt surface sites. The modified electronic structure of the alloyed Pt controlled the probability of the C–O bond scission. The bimetallic clusters restructured during the reaction such that the Au atoms in the clusters aggregated and decorated the Pt surface, leading to fewer surface Pt and increased mean coordination of surface Au.

 Please wait while we load your content...
Please wait while we load your content...