Bioactive compounds derived from echinoderms
Abstract
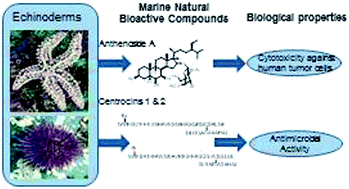
The marine environment provides a rich source of natural products with potential therapeutic applications. The rate of studies in marine animals, particularly invertebrates has increased considerably in the last few years leading to an increase in the number of bioactive compounds discovered. In this context, this review focuses on the phylum Echinodermata and aims at summarizing and highlighting the bioactive compounds derived from the echinoderms discovered between 2009 and 2013, clarifying their structure, distribution, biosynthetic origin, and biological activity.

 Please wait while we load your content...
Please wait while we load your content...