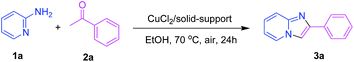Heterogeneously copper-catalyzed oxidative synthesis of imidazo[1,2-a]pyridines using 2-aminopyridines and ketones under ligand- and additive-free conditions†
Xu Meng,
Yanmin Wang,
Chaoying Yu and
Peiqing Zhao*
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, P. R China. E-mail: zhaopq@licp.cas.cn; Fax: +86 931 8277008; Tel: +86 931 4968688
First published on 29th May 2014
Abstract
An efficient and mild heterogeneously CuCl2/nano-TiO2-catalyzed aerobic synthesis of imidazo[1,2-a]pyridines from 2-aminopyridines and ketones has been developed using air as the oxidant in the absence of any ligands and additives. This strategy was compatible with a large range of substrates, including unactivated aryl ketones and unsaturated ketones and went through the C–H bond functionalization mechanism instead of I−-assisted Ortoleva-King reaction to provide the corresponding imidazo[1,2-a]pyridines in good yields with low catalyst loading (0.8 mol%). Moreover, the heterogeneous catalyst can be successfully employed in gram-scale synthesis and reused many times without the significant loss of catalytic activity.
Introduction
Due to increasing concern about environmental and economic issues, significant effort has been made to develop new catalytic methods, which minimize pollution and are suited for application in industrial processes, such as heterogeneous catalysis.1 Compared with homogeneous catalysis, its heterogeneous counterpart has practical advantages in catalyst handling, recyclability and separation of the catalyst from products, which results in its wide applications in industrial environments.2–6 Furthermore, heterogeneous catalysis meets the requirements of green chemistry because it employs recycled catalysts and ligand-free conditions generally.4,6 Therefore, it is highly desirable to explore economic and eco-friendly heterogeneous catalytic systems for organic synthesis.Imidazo[1,2-a]pyridines are valuable motifs and exhibit many important biological activities, like antibacterial,7 antiviral,8 antitumor9 and anti-inflammatory properties.10 Therefore, imidazo[1,2-a]pyridines are widely present in a number of best-selling pharmaceuticals,11 such as zolimidine,12 zolpidem,12 alpidem,13 olprinone,14 necopidem,15 saripidem.15 Traditionally, imidazo[1,2-a]pyridines were obtained by condensations between 2-aminopyridines and per-functionalized carbonyl compounds under various conditions (Scheme 1, eqn (1)).16 Recently, Lei's group discovered a silver-mediated oxidative coupling/cyclization using 2-aminopyridines and terminal alkynes to provide imidazo[1,2-a]pyridines (Scheme 1, eqn (2)).17
In 2013, Hajra's group reported a Fe-catalyzed method using 2-aminopyridines and nitroolefins (Scheme 1, eqn (3)).18 Subsequently, several homogeneous CuI or Cu/ZnI-catalyzed oxidative methods using 2-aminopyridines and unactivated methyl ketones in the presence of ligands or additives were reported, respectively, by Hajra, Adimurthy, Ji and Kumar's group at almost the same time (Scheme 1, eqn (4)).19 Most recently, Su and co-workers discovered a homogeneous CuI/O2 system for the synthesis of imidazo[1,2-a]pyridines by a reaction between 2-aminopyridines and unactivated methyl ketones and disclosed an iodine-promoted Ortoleva-King reaction rather than the previously reported C–H functionalization, which was involved in this transformation most probably (Scheme 1, eqn (4)).20 In the course of investigating applications of heterogeneous catalysts in our research group,4a,e,21 we proposed a simple heterogeneous system for the synthesis of imidazo[1,2-a]pyridines from unactivated ketones and 2-aminopyridines via metal-catalyzed aerobic C–H functionalization under ligand-, additive- and iodine-free conditions, which would be a useful complement to the previous methodologies.22
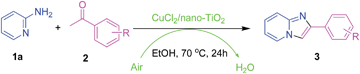
Here, we wish to describe a heterogeneous CuCl2/nano-TiO2-catalyzed aerobic synthesis of imidazo[1,2-a]pyridines from 2-aminopyridines and readily available ketones using air as the oxidant under iodine-, ligand- and additive-free conditions (Scheme 2). The catalytic system tolerates a variety of substrates and could be employed in the synthesis of zolimidine on a gram-scale. More importantly, the heterogeneous catalyst can recycle three times without the loss of activity.
Results and discussion
Initially, a series of bimetallic heterogeneous catalysts which are successfully used in the aerobic synthesis of 1,2,4-triazoles4e were employed for the catalytic synthesis of imidazo[1,2-a]pyridines in DCB (1,2-dichlorobenzene) under air at the indicated temperatures (Table 1, entries 1–7). Although the desired product was obtained in very low yield only at 130 °C for 24 h using Cu–Zn/nano-TiO2, most experiments did not proceed well. Then, we focused on a copper-based solid-supported catalyst and delightedly found that CuCl2/nano-TiO2 (0.8 mol%, Cu = 3.16 wt%) could catalyze the cyclization efficiently while copper hydroxide and oxide were both inferior at 130 °C for 24 h (Table 1, entries 9, 10, 14 and 15).| Entry | Catalyst | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 2-aminopyridine (0.6 mmol), acetophenone (0.5 mmol), heterogeneous catalyst (12 mg, 0.8 mol%), solvent (0.6 mL), air, 24 h.b Isolated yields.c 10 mol% of CuCl2-2H2O was used.d 12 mg of nano-TiO2 was used.e 10 mol% of CuCl2-2H2O and 12 mg of nano-TiO2 were used. | ||||
| 1 | Cu–Zn/Al–Ti | DCB | 100 | 0 |
| 2 | Cu–Zn/Al–Ti | DCB | 130 | <5 |
| 3 | Cu–Mn/Al–Ti | DCB | 120 | 0 |
| 4 | Fe–Zn/Al–Ti | DCB | 120 | 0 |
| 5 | Cu–Zn/nano-TiO2 | DCB | 130 | 8 |
| 6 | Cu–Ni/nano-TiO2 | DCB | 130 | <5 |
| 7 | Ni–Zn/nano-TiO2 | DCB | 130 | 0 |
| 8 | Cu–Fe/OMS-2 | DCB | 130 | <5 |
| 9 | Cu(OH)x/OMS-2 | DCB | 130 | <5 |
| 10 | CuCl2/nano-TiO2 | DCB | 130 | 88 |
| 11c | CuCl2 | DCB | 130 | 12 |
| 12d | Nano-TiO2 | DCB | 130 | 0 |
| 13e | CuCl2 + nano-TiO2 | DCB | 130 | 15 |
| 14 | Cu(OH)x/nano-TiO2 | DCB | 130 | 23 |
| 15 | CuOx/nano-TiO2 | DCB | 130 | 0 |
| 16 | Ru/nano-TiO2 | DCB | 130 | 0 |
| 17 | Pt/nano-TiO2 | DCB | 130 | 0 |
| 18 | CuCl2/nano-TiO2 | DMSO | 130 | 16 |
| 19 | CuCl2/nano-TiO2 | DMF | 130 | <5 |
| 20 | CuCl2/nano-TiO2 | o-Xylene | 130 | 12 |
| 21 | CuCl2/nano-TiO2 | Dioxane | 100 | 65 |
| 22 | CuCl2/nano-TiO2 | MeNO2 | 100 | <5 |
| 23 | CuCl2/nano-TiO2 | THF | 60 | <5 |
| 24 | CuCl2/nano-TiO2 | EtOH | 70 | 87 |
The supported Cu(OH)x on manganese oxide-based octahedral molecular sieve (Cu(OH)x/OMS-2), which is an excellent heterogeneous catalyst for biomimetic homo-coupling of terminal alkynes,23a and basic Cu(OH)x/nano-TiO2, which can successfully catalyze Huisgen [3 + 2] cycloaddition,23b are not efficient for this cyclization, although the latter gave 23% yield (Table 1, entries 9 and 14). On the other hand, the copper oxide does not have catalytic activity at all in the experiment (Table 1, entry 15). More experiments showed that no reaction proceeded or low yield was obtained in the presence of nano-TiO2 powder alone or the catalyst precursor of CuCl2-2H2O (Table 1, entries 11 and 12). Moreover, the catalytic activity of a mixture of CuCl2-2H2O and nano-TiO2 was much lower than that of the CuCl2/nano-TiO2 (Table 1, entry 13), which means that the highly dispersed copper species are effective for this cyclization. Specifically, CuCl2 can catalyze the reaction while nano-TiO2 does not have any catalytic activity; therefore, nano-TiO2 probably plays a role of an appropriate support for the heterogeneous catalyst because of its large surface area, small particle size and the interaction between Cu species and TiO2.24 To improve the yield of reaction, other metal oxides were used, such as Ru and Pt, although the results were negative (Table 1, entries 16 and 17). Next, we found that the solvent could affect the reaction significantly (Table 1, entries 18–24). Specifically, polar organic solvents, like DMF and DMSO, hardly assisted with the reaction while only dioxane brought about moderate yield of the desired product among other examined solvents (Table 1, entries 18–23). Surprisingly, the best result was obtained by using protic EtOH as a solvent under mild conditions (Table 1, entry 24).
After confirming the catalytic transition metal, a lot of supports were examined for optimization as well (Table 2). In Table 2, we used the impregnation method to load CuCl2 on various solid supports and ran the cyclization under the established reaction conditions: EtOH as a solvent at 70 °C under air for 24 h. As a consequence, nano-size Al2O3, ZrO2 and CeO2 were not efficient supports, although they catalyzed the reaction slightly (Table 2, entries 2–4). SiO2, OMS-2 (manganese oxide-based octahedral molecular sieve), activated carbon and ATP (attapulgite) gave traces to approximately 10% yield (Table 2, entries 5–7 and 9) while CuCl2/Al–Ti gave moderate yield of the desired product (Table 2, entry 8). Finally, it was proven that nano-TiO2 is the best support for the heterogeneous catalyst under mild ligand- and additive-free conditions (Table 2, entry 1).
| Entry | Catalyst (0.8 mol%) | Isolated yield (%) |
|---|---|---|
| a Reaction conditions: 2-aminopyridine (0.6 mmol), acetophenone (0.5 mmol), heterogeneous catalyst (12 mg, 0.8 mol%), EtOH (0.6 mL), 70 °C air, 24 h. | ||
| 1 | CuCl2/nano-TiO2 | 87 |
| 2 | CuCl2/nano-Al2O3 | 15 |
| 3 | CuCl2/nano-ZrO2 | 26 |
| 4 | CuCl2/nano-CeO2 | 10 |
| 5 | CuCl2/SiO2 | 8 |
| 6 | CuCl2/OMS-2 | 11 |
| 7 | CuCl2/C | <5 |
| 8 | CuCl2/Al–Ti | 34 |
| 9 | CuCl2/ATP | <5 |
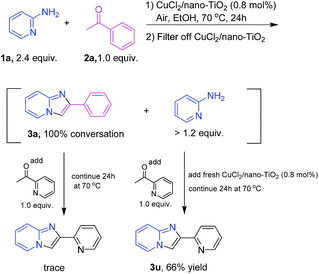
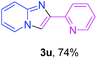
To verify whether the observed catalysis is derived from the solid catalyst CuCl2/nano-TiO2 or the leached copper species, the cyclization of 1a and 2a was carried out, and then the catalyst was removed after filtering a totally converted reaction mixture (acetophenone as a substrate). Next, 1.0 equiv. of a different ketone (2-acetylpyridine) was added as an additional substrate to the filtrate, and then the filtrate was treated with the remaining amount of 2-aminopyridine (>1.2 equiv.). Consequently, only trace amounts of another product 3u was discovered, while 66% yield of 3u was obtained, if the fresh CuCl2/nano-TiO2 was added to the filtrate (Scheme 3). ICP-AES was used to analyze the filtrate that was removed by CuCl2/nano-TiO2, which confirmed that no copper species was detected in the filtrate (Cu: below 0.001%). These results indicated that the catalysis is from the solid catalyst rather than the leached metal species, and the catalyst is unambiguously heterogeneous in nature.
After the cyclization was completed, CuCl2/nano-TiO2 was easily retrieved from the reaction mixture by centrifugation and filtration. The retrieved catalyst could be reused at least three times with a slight decrease in catalytic ability (Table 3).
| Cycle | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| a Reaction conditions: 2-aminopyridine (0.6 mmol), acetophenone (0.5 mmol), CuCl2/nano-TiO2 (12 mg, 0.8 mol%), ethanol (0.6 mL), air, 70 °C, 24 h. | ||||
| Isolated yield of 3b (%) | 87 | 79 | 72 | 53 |
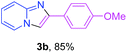
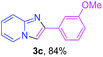
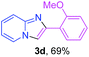
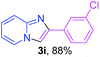
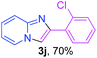
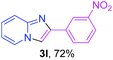
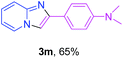
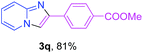
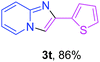
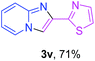
With the optimized conditions in hand, we sought to define the scope of the aryl ketones using 2-aminopyridine 1a as the substrate. As shown in Table 4, electron-rich and -poor aryl ketones could undergo cyclizations with 2-aminopyridine successfully, including sensitive NO2-substituted acetophenone. Specifically, electron-poor ketones could give the corresponding products in excellent yields, while electron-poor ketones offered relatively low yields of imidazo[1,2-a]pyridines. Moreover, steric hindrance could influence the reaction, which made ortho-substituted aryl ketones give moderate yields of the desired products (Table 4, 3d, 3j and 3p). Interestingly, the ester group and N,N-dimethyl-substituted ketones also performed smoothly (Table 4, 3m and 3q). Remarkably, cyano was tolerated in the cyclization chemoselectively and offered excellent yield of imidazo[1,2-a]pyridine rather than 1,2,4-triazole under Cu-catalyzed aerobic oxidative conditions (Table 4, 3r).25 Finally, heteroaromatic ketones, such as furan, thiophene, pyridine and thiazole, were effective substrates and provided good yields respectively (Table 4, 3s–v).
| a Reaction conditions: 1a (0.6 mmol), 2 (0.5 mmol), CuCl2/nano-TiO2 (0.8 mol%, 12 mg), EtOH (0.6 mL), air, 70 °C, 24 h, isolated yields. | ||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
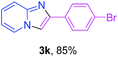 |
 |
 |
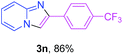 |
 |
 |
 |
 |
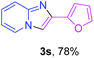 |
 |
 |
 |
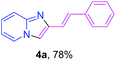
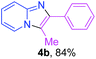
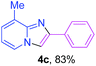
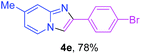
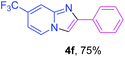
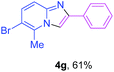
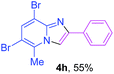
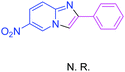
Next, more efforts were made to further explore the scope of the heterogeneous cyclization. In Table 5, alkenyl-substituted imidazo[1,2-a]pyridine, which was obtained with difficulty by previous methods,16m,n was synthesized by this simple heterogeneous method using α,β-unsaturated ketone (Table 5, 4a). Furthermore, propiophenone was also a successful substrate for the reaction (Table 5, 4b). Subsequently, various substituted 2-aminopyridines were investigated and provided moderate to good yields (Table 5, 4c–h), including CF3- and multi-substituted 2-aminopyridines. Unfortunately, nitro substituted 2-aminopyridine could not participate in the cyclization (Table 5).
| a Reaction conditions: 1 (0.6 mmol), 2 (0.5 mmol), CuCl2/nano-TiO2 (12 mg, 0.8 mol%), EtOH (0.6 mL), 70 °C, 24 h, air, isolated yields. | ||
|---|---|---|
 |
 |
 |
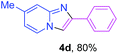 |
 |
 |
 |
 |
 |
To demonstrate the operational simplicity and broad generality of this heterogeneous protocol, a gram-scale reaction was carried out using 2-aminopyridine and 4-methylsufonyl acetophenone under slightly modified reaction conditions (Scheme 4). The synthesis of the marketed drug zolimidine 5 was achieved on a gram-scale with 56% isolated yield under heterogeneous CuCl2/nano-TiO2-catalyzed ligand- and additive-free conditions.
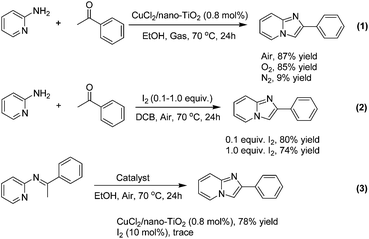
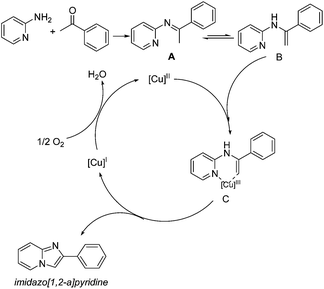
Finally, some control experiments were conducted to further understand the mechanism of this heterogeneous copper-catalyzed oxidative cyclization (Scheme 5). The cyclizations were carried out under different atmospheres and results showed that the products were obtained under air or O2, while it failed to yield the desired product efficiently under N2, which indicated that the oxygen plays a role of an oxidant in the synthesis of imidazo[1,2-a]pyridines (Scheme 5, eqn (1)). According to the previous methods of homogeneous Cu-catalyzed synthesis of imidazo[1,2-a]pyridines from 2-aminopyridines and ketones,19 especially Su's latest work on CuI-catalysis,20 it is reasonable that the reaction mechanism of the previous copper-catalysis involved a catalytic Ortoleva-King reaction16g more probably rather than the copper-catalyzed C–H functionalization because of the use of iodine in the previous methods.22g,26 To prove this proposal, the cyclizations were conducted directly using I2 without any copper source (Scheme 5, eqn (2)). Interestingly, the cyclizations preformed smoothly no matter how much I2 was used in the reaction, which means that a-iodoacetophenone rather than imine is formed firstly for further Ortoleva-King reaction and copper does not play a crucial role when the cyclization is catalyzed by CuI or Cu/ZnI systems. More importantly, when the imine formed from 2-aminopyridine and acetophenone was employed as a starting substrate, the desired product was obtained in 78% yield under a heterogeneous CuCl2/nano-TiO2 system. On the other hand, the reaction failed when a catalytic amount of I2 was used as a catalyst (Scheme 5, eqn (3)). Under our optimized conditions, the absence of iodine anion rules out the possibility of the Ortoleva-King reaction in terms of mechanism.
Based on the observations and summary from control experiments and the works of Su and Han's group,20,27 a plausible mechanism involving oxidative C–H functionalization is postulated in Scheme 6.19 Firstly, imine A was generated via the condensation of 2-aminopyridine and acetophenone. Then, enamine B was formed by tautomerization of imine A.28 Subsequently, intermediate C was formed via oxidative addition between Cu(II) species and enamine B under the present conditions.29 After reductive elimination, the desired product imidazo[1,2-a]pyridine was given along with the reduced Cu(I) species, which was oxidized by oxygen to finish the catalytic cycle.
Conclusions
In summary, we have developed a heterogeneous copper-catalyzed synthesis of imidazo[1,2-a]pyridines from 2-aminopyridines and unactivated ketones in the absence of iodine anion, ligands and additives. The heterogeneous catalytic system which uses air as an efficient oxidant can tolerate a large range of substrates under mild conditions and provide desired products in good yields. Moreover, the present heterogeneous method can be applied in one-step synthesis of zalimidine on a gram scale and the heterogeneous catalyst can be recycled many times. With respect to the mechanism, the cyclization more likely undergoes a Cu-catalyzed oxidative C–H bond functionalization instead of the previously reported iodine anion catalyzed Ortoleva-King reaction under these heterogeneous conditions. Further studies to fully understand the mechanism of the reaction and the application of the CuCl2/nano-TiO2 in other transformations are in progress in our lab.Experimental
General
All reagents were purchased from commercial suppliers and used without further purification. Metal salts and catalyst supports were commercially available and were directly used. All experiments were carried out under air. Flash chromatography was carried out using Merck silica gel 60 (200–300 mesh). Analytical TLC was performed using Merck silica gel 60 F254 plates, and the products were visualized by UV detection. 1H NMR and 13C NMR (400 and 100 MHz respectively) spectra were recorded in CDCl3. Chemical shifts (δ) are reported in ppm using TMS as an internal standard, and spin–spin coupling constants (J) are given in Hz.Preparation of CuCl2/nano-TiO223b
Support nano-TiO2 powder (1 g, anatase, particle size ≤ 30 nm, BET surface area ≥ 120 m2 g−1) was added to a 50 mL round-bottom flask. A solution of CuCl2-2H2O (0.108 g) in acetone (10 mL) was added to nano-TiO2 powder, and additional acetone (10 mL) was added to wash down the sides of the flask. Then the flask was submerged into an ultrasound bath for 3 h at room temperature and stirred for further 20 h at room temperature. Furthermore, the acetone was distilled under reduced pressure on a rotary evaporator at 50 °C for more than 2 h. Finally, a dried pale green powder was obtained. The inductively coupled plasma optical emission spectrum (ICP-OES) showed the Cu content to be 3.16 wt%.General procedure for CuCl2/nano-TiO2-catalyzed cyclization
CuCl2/nano-TiO2 (12 mg, 0.8 mol%), 2-aminopyridine (0.6 mmol), ketone (0.5 mmol) and EtOH (0.6 mL) were added to a flask with a bar. The flask was stirred at 70 °C for 24 h under air. After cooling to room temperature, the mixture was diluted with ethyl acetate and filtered. The filtrate was removed under reduced pressure to get the crude product, which was further purified by silica gel chromatography (petroleum![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 3
ethyl acetate = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as an eluent) to yield the corresponding product. The identity and purity of the products was confirmed by 1H and 13C NMR spectroscopic analysis.
1 as an eluent) to yield the corresponding product. The identity and purity of the products was confirmed by 1H and 13C NMR spectroscopic analysis.
Notes and references
- (a) H. U. Blaser and E. Schmidt, Asymmetric Catalysis on Industrial Scale, Wiley-VCH, Weinheim, 2004 Search PubMed; (b) R. A. Sheldon and H. van Bekkum, Fine Chemicals through Heterogeneous Catalysis, Wiley-VCH, Weinheim, 2001 Search PubMed; (c) E.-J. Ras and G. Rothenberg, RSC Adv., 2014, 4, 5963 RSC.
- A. Thayer, Chem. Eng. News, 2005, 83, 55 Search PubMed.
- Review on heterogeneous palladium-catalyzed couplings: L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133 CrossRef CAS PubMed.
- For the selected papers on the application of solid-supported heterogeneous catalysts, see: (a) X. Meng, X. Xu, T. Gao and B. Chen, Eur. J. Org. Chem., 2010, 5409 CrossRef CAS; (b) R. B. N. Baig and R. S. Varma, Green Chem., 2013, 15, 1838 RSC; (c) D. D. Tang, K. D. Collins and F. Glorius, J. Am. Chem. Soc., 2013, 135, 7450 CrossRef CAS PubMed; (d) C. Liu, X. Rao, Y. Zhang, X. Li, J. Qiu and Z. Jin, Eur. J. Org. Chem., 2013, 4345 CrossRef; (e) X. Meng, C. Yu and P. Zhao, RSC Adv., 2014, 4, 8612 RSC; (f) J. B. Bharate, S. K. Guru, S. K. Jain, S. Meena, P. P. Singh, S. Bhushan, B. Singh, S. B. Bharate and R. A. Vishwakarma, RSC Adv., 2013, 3, 20869 RSC.
- For the selected reviews on the application of nanocatalysts, see: (a) E. Gross, J. H.-C. Liu, F. D. Toste and G. A. Somorjai, Nat. Chem., 2012, 4, 947 CrossRef CAS PubMed; (b) V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed; (c) K. V. S. Ranganath and F. Glorius, Catal. Sci. Technol., 2011, 1, 13 RSC; (d) A. Schātz, O. Reiser and W. J. Stark, Chem.–Eur. J., 2010, 16, 8950 CrossRef PubMed.
- Selective reviews on heterogeneous catalysis, see: (a) M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072 CrossRef CAS PubMed; (b) M. Sankar, N. Dimitratos, P. J. Miedziak, P. P. Wells, C. J. Kiely and G. J. Hutchings, Chem. Soc. Rev., 2012, 41, 8099 RSC; (c) M. J. Climent, A. Corma and S. Iborra, RSC Adv., 2012, 2, 16 RSC; (d) N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS.
- (a) M. H. Fisher and A. Lusi, J. Med. Chem., 1972, 15, 982 CrossRef CAS PubMed; (b) J. C. Teulade, G. Grassy, J. P. Girard and J. P. Chapat, Eur. J. Med. Chem., 1978, 13, 271 CAS.
- (a) A. Gueiffier, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chaxignon, J. C. Teulade, A. Kerbal, E. M. Essassi, J. C. Debouzy, M. Witvrouw, Y. Blache, J. Balzarini, E. De Clercq and J. P. Chapat, J. Med. Chem., 1996, 39, 2856 CrossRef CAS PubMed; (b) M. Lhassani, O. Chavignon, J. M. Chezal, J. C. Teulade, J. P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. De Clercq and A. Gueiffier, Eur. J. Med. Chem., 1999, 34, 271 CrossRef CAS.
- (a) E. Badaway and T. Kappe, Eur. J. Med. Chem., 1995, 30, 327 CrossRef; (b) M. Hranjec, I. Piantanida, M. Kralj, L. Suman, K. Pavelıć and G. Karminski-Zamola, J. Med. Chem., 2008, 51, 4899 CrossRef CAS PubMed; (c) M. Hranjec, M. Kralj, I. Piantanida, M. Sedıć, L. Suman, K. Pavelıć and G. Karminski-Zamola, J. Med. Chem., 2007, 50, 5696 CrossRef CAS PubMed.
- C. Hamdouchi, J. de Blas, M. del Prado, J. Gruber, B. A. Heinz and L. Vance, J. Med. Chem., 1999, 42, 50 CrossRef CAS PubMed.
- M. Baumann, I. R. Baxendale, S. V. Ley and N. Nikbin, Beilstein J. Org. Chem., 2011, 7, 442 CrossRef CAS PubMed.
- L. Almirante, L. Polo, A. Mugnaini, E. Provinciali, P. Rugarli, A. Biancotti, A. Gamba and W. Murmann, J. Med. Chem., 1965, 8, 305 CrossRef CAS PubMed.
- S. Z. Langer, S. Arbilla, J. Benavides and B. Scatton, Adv. Biochem. Psychopharmacol., 1990, 46, 61 CAS.
- K. Mizushige, T. Ueda, K. Yukiiri and H. Suzuki, Cardiovasc. Drug Rev., 2002, 20, 163 CrossRef CAS PubMed.
- R. J. Boerner and H. J. Moller, Psychopharmakotherapie, 1997, 4, 145 Search PubMed.
- (a) E. S. Hand and W. W. Paudler, J. Org. Chem., 1978, 43, 2900 CrossRef CAS; (b) N. Denora, V. Laquintana, M. G. Pisu, R. Dore, L. Murru, A. Latrofa, G. Trapani and E. Sanna, J. Med. Chem., 2008, 51, 6876 CrossRef CAS PubMed; (c) D. Zhu, J. Chen, D. Wu, M. Liu, J. Ding and H. Wu, J. Chem. Res., 2009, 2, 84 CrossRef; (d) D.-J. Zhu, J.-X. Chen, M.-C. Liu, J.-C. Ding and H.-Y. Wu, J. Braz. Chem. Soc., 2009, 20, 482 CrossRef CAS; (e) S. E. Kazzouli, S. Berteina-Raboin, A. Mouaddib and G. Guillaumet, Tetrahedron Lett., 2003, 44, 6265 CrossRef; (f) M. Ueno, T. Nabana and H. Togo, J. Org. Chem., 2003, 68, 6424 CrossRef CAS; (g) A. J. Stasyuk, M. Banasiewicz, M. K. Cyrański and D. T. Gryko, J. Org. Chem., 2012, 77, 5552 CrossRef CAS; (h) T. J. Donohoe, M. A. Kabeshov, A. H. Rathi and I. E. D. Smith, Org. Biomol. Chem., 2012, 10, 1093 RSC; (i) Z.-G. Le, Z.-B. Xie and J.-P. Xu, Molecules, 2012, 17, 13368 CrossRef CAS; (j) J. S. Yadav, B. V. Subba Reddy, Y. Gopal Rao, M. Srinivas and A. V. Narsaiah, Tetrahedron Lett., 2007, 43, 7717 CrossRef; (k) Y.-Y. Xue, Z.-C. Chen and Q.-G. Zheng, Synthesis, 2002, 11, 1505 Search PubMed; (l) S. Kumar and D. P. Sahu, ARKIVOC, 2008, xv, 88 Search PubMed; (m) A. R. Katritzky, D. O. Tymoshenko, D. Monteux, V. Vvedensky, G. Nikonov, C. B. Cooper and M. Deshpande, J. Org. Chem., 2000, 65, 8059 CrossRef CAS; (n) R. J. Sundberg, D. J. Dahlhausen, G. Manikumar, B. Mavunkel, A. Biswas, V. Srinivasan, K. King and P. Waid, J. Heterocycl. Chem., 1988, 25, 129 CrossRef CAS.
- C. He, J. Hao, H. Xu, Y. Mo, H. Liu, J. Han and A. Lei, Chem. Commun., 2012, 48, 11073 RSC.
- S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065 CrossRef CAS.
- (a) A. K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741 CrossRef CAS; (b) D. C. Mohan, R. R. Donthiri, S. N. Rao and S. Adimurthy, Adv. Synth. Catal., 2013, 355, 2217 CrossRef; (c) Z.-J. Cai, S.-Y. Wang and S.-J. Ji, Adv. Synth. Catal., 2013, 355, 2686 CrossRef CAS; (d) K. Pericherla, P. Kaswan, P. Khedar, B. Khungar, K. Parang and A. Kumar, RSC Adv., 2013, 3, 18923 RSC.
- Y. Zhang, Z. Chen, W. Wu, Y. Zhang and W. Su, J. Org. Chem., 2013, 78, 12494 CrossRef CAS.
- (a) J. Liu, C. Yu, P. Zhao and G. Chen, Appl. Surf. Sci., 2012, 258, 9096 CrossRef CAS; (b) C. Yu, P. Zhao, G. Chen and B. Hu, Appl. Surf. Sci., 2011, 257, 7727 CrossRef CAS; (c) X. Meng, X. Li, W. Chen, Y. Zhang, W. Wang, J. Chen, J. Song, H. Feng and B. Chen, J. Heterocycl. Chem., 2014, 51, 349 CrossRef CAS.
- Selective recent papers on homogeneous synthesis of imidazopyridines using non-ketones/2-aminopyridine substrates, see: (a) E. P. A. Talbot, M. Richardson, J. M. McKenna and F. D. Toste, Adv. Synth. Catal., 2014, 356, 687 CrossRef CAS; (b) H. Huang, X. Ji, X. Tang, M. Zhang, X. Li and H. Jiang, Org. Lett., 2013, 15, 6254 CrossRef CAS; (c) J. Yu, Y. Jin, H. Zhang, X. Yang and H. Fu, Chem.–Eur. J., 2013, 19, 16804 CrossRef CAS; (d) K. B. Puttaraju and K. Shivashankar, RSC Adv., 2013, 3, 20883 RSC; (e) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS; (f) I. R. Siddiqui, P. Rai, Rahila, A. Srivastava and S. Shamim, Tetrahedron Lett., 2014, 55, 1159 CrossRef CAS; (g) Z. Fei, Y. Zhu, M. Liu, F. Jia and A. Wu, Tetrahedron Lett., 2013, 54, 1222 CrossRef CAS.
- (a) T. Oishi, K. Yamaguchi and N. Mizuno, ACS Catal., 2011, 1, 1351 CrossRef CAS; (b) K. Yamaguchi, T. Oishi, T. Katayama and N. Mizuno, Chem.–Eur. J., 2009, 15, 10464 CrossRef CAS.
- (a) H. Irie, S. Miura, K. Kamiya and K. Hashimoto, Chem. Phys. Lett., 2008, 457, 202 CrossRef CAS; (b) G. Li, N. M. Dimirijevic, L. Chen, T. Rajh and K. A. Gray, J. Phys. Chem. C, 2008, 112, 19040 CrossRef CAS.
- S. Ueda and H. Nagasawa, J. Am. Chem. Soc., 2009, 131, 15080 CrossRef CAS.
- Selective papers on formation of a-iodoketones using I- and ketones, see: (a) Y.-P. Zhu, J.-J. Yuan, Q. Zhao, M. Lian, Q.-H. Gao, M.-C. Liu, Y. Yang and A.-X. Wu, Tetrahedron, 2012, 68, 173 CrossRef CAS; (b) Q.-H. Gao, Z. Fei, Y.-P. Zhu, M. Lian, F.-C. Jia, M.-C. Liu, N.- She and A.-X. Wu, Tetrahedron, 2013, 69, 22 CrossRef CAS; (c) W.-J. Xue, H.-Z. Li, F.-F. Gao and A. Wu, Tetrahedron, 2014, 70, 239 CrossRef CAS.
- L. Ma, X. Wang, W. Yu and B. Han, Chem. Commun., 2011, 47, 11333 RSC.
- Y. Wei, I. Deb and N. Yoshikai, J. Am. Chem. Soc., 2012, 134, 9098 CrossRef CAS.
- (a) W. Zhou, Y. Liu, Y. Yang and G.-J. Deng, Chem. Commun., 2012, 48, 10678 RSC; (b) H. Wang, Y. Wang, C. Peng, J. Zhang and Q. Zhu, J. Am. Chem. Soc., 2010, 132, 13217 CrossRef CAS; (c) S. V. Ley and A. W. Thoms, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (d) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03299c |
| This journal is © The Royal Society of Chemistry 2014 |