CH2Cl2 as reagent in the synthesis of methylene-bridged 3,3′-bis(oxazolidin-2-one) derivatives under ambient conditions†
Qingfeng Liu,
Yansen Zhang,
Zhiguo Zhang,
Tongxin Liu,
Lei Shi and
Guisheng Zhang*
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P.R. China. E-mail: zgs6668@yahoo.com; Fax: +86 373-3325250
First published on 23rd May 2014
Abstract
This paper describes a highly efficient and facile transformation of substituted oxazolidin-2-ones to methylene-bridged 3,3′-bis(oxazolidin-2-one) derivatives in a mixture solvent of CH2Cl2 (DCM) and N,N-dimethylformamide (DMF) in the presence of NaH at ambient temperature. Isotopic labeling experiments indicated that the C of the bridged methylene group was derived from CH2Cl2 instead of DMF and other unknown substances in the NaH reagent (60% dispersion in mineral oil).
Typically, inert DCM is chosen as a solvent for its versatility in a variety of organic applications. The activation of DCM has been studied because of an interest in the construction of methylene-bridged bisamines, which are usually used as various ligands,1 N-heterocyclic carbenes.2 DCM has been reported to react with aliphatic amines,3–6 i.e. the nitrogen-containing heterocyclic compounds, such as imidazole,2,7 pyrrolidine,8 pyrrole,9 pyrazole,10 pyridine,11 and 1,2,3-triazole,12 to form the corresponding methylene-bridged compounds with moderate to high yields. However, due to their low reactivity, the reaction of oxazolidin-2-ones with DCM to form methylene-bridged bis(2-oxazolidinone) derivatives has not been realized yet.
2-Oxazolidinones have been widely utilized in asymmetric reactions, such as aldol condensation,13 alkylation,14 halogenation,15 amination,16 radical addition,17 and Diels–Alder reactions,18 and as intermediates for the asymmetric synthesis of bioactive compounds.13c,19 Moreover, a relatively large number of naturally occurring and synthetic molecules with antibacterial, antiallergenic, immunosuppressants, or monoamine oxidase inhibition activities contain the amino alcohol functionality.20 Recently, we designed a series of chiral compounds that can be prepared from the intermediate methylene-bridged 3,3′-bis((S)-4-benzyloxazolidin-2-one) (2a, Table 1) to evaluate their antitumor activity. For the preparation of the key intermediate 2a, we first attempted to follow the reported methods,7–12 which were successfully applied to prepare other methylene-bridged bis(N-heterocycle)s via the reactions of DCM with imidazole,2,7 pyrrolidine,8 pyrrole,9 pyrazole,10 pyridine,11 and 1,2,3-triazole.12 To our disappointment, these methods did not work to prepare 2a. A few synthetic examples of methylene-bridged 3,3′-bis(oxazolidin-2-one) derivatives have been reported to date. Ingleby21 reported an efficient approach to 3,3′-bis(oxazolidin-2-one) methanes via the reaction of oxazolidin-2-ones with paraformaldehyde in the presence of p-MeC6H4SO3H at 100 °C; Zinner22 demonstrated two procedures to prepare bis(benzoxazolidinon-3-yl)methane by treating benzoxazolidinone with benzoxazolon-3-ylchloromethane and metal sodium in EtOH under refluxing, and with CH2Br2 catalyzed by KOH in EtOH in a water bath in yields of 60%, and 9%, respectively. Seebach23 treated 4-isopropyl-5,5-diphenyloxazolidinone with BuLi, NaI and chloromethyl methyl sulfide in DME at 0 °C to obtain the dimer 3,3′-bis((S)-4-isopropyl-5,5-diphenyl-2-oxazolidinon-3-yl)methane as a side product in a yield of 13%. To the best of our knowledge, there is no detailed investigation on the synthesis of bis(2-oxazolidinon-3-yl) methanes, particularly under mild conditions. Herein, we report a mild and efficient approach to bis(2-oxazolidinon-3-yl)methanes by treating 2-oxazolones with NaH in mixture solvents of DCM and DMF under ambient temperature, in which DCM is disclosed as a C source.
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise indicated, all the reactions were carried out with 1a (1 mmol) and NaH (1 equiv.) in a solvent (3 mL) at ambient temperature (25–30 °C).b Isolated yield of 2a.c 1.5 equiv. of NaH was used.d 1.8 equiv. of NaH was used. | |||
| 1 | DCM | 48 | 0 |
| 2 | DMF | 48 | 0 |
| 3 | DCM/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
33 | 46 |
| 4 | DCM/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
33 | 28 |
| 5 | DCM/EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
48 | 0 |
| 6 | DCM/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
48 | 0 |
| 7 | DCM/Et2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
48 | 0 |
| 8 | DCM/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
27 | 61c |
| 9 | DCM/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
9 | 98d |
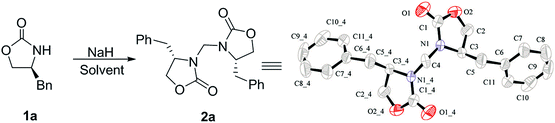
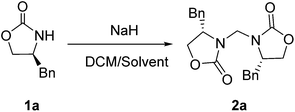
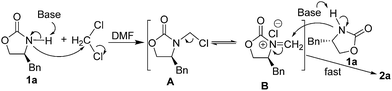
During the course of our ongoing work on the synthesis of oxazolidinone derivatives, we performed a reaction of (S)-4-benzyloxazolidin-2-one (1a) with an alkynyl bromide mediated by NaH in DMF. The reaction was conducted by stirring a mixture of (S)-4-benzyloxazolidin-2-one and NaH in DMF for a certain time. Subsequently, the alkynyl bromide, which was dissolved in a small amount of DCM, was added to the mixture, and the resulting reaction mixture was then stirred for a certain time under ambient temperature. During the work-up of the reaction mixture, a small amount of an unexpected byproduct was accidentally separated, which was subsequently characterized as a methylene-bridged 3,3′-bis(oxazolidin-2-one) derivate 2a by 1H NMR, 13C NMR, HRMS and X-ray diffraction (XRD) analysis. Because of the mild and basic condition, this unexpected finding is worthy of further evaluation as an alternative and efficient approach to 3,3′-bis(oxazolidin-2-one)methanes 2. This finding suggested that DCM should be a reagent to form 2a. Therefore, to optimize this reaction, a reaction of 1a in pure DCM was first performed (Table 1, entry 1), to our surprise, no reaction occurred. This result raises doubt as to whether DMF would provide the bridged carbon of 2a because DMF can also serve as a carbon source.24 However, the reaction of 1a in pure DMF also did not work (Table 1, entry 2). Subsequently, some mixed solvents, such as DCM/DMF, DCM/THF, and DCM/Et2O, were screened (Table 1, entries 3–7), and the results indicated that both DCM and DMF are necessary for the reaction. Using this result, a detailed screening of the reaction conditions was conducted using the model compound of 1a. After several attempts, the optimal condensation condition was developed, which is listed in Table 1 (entry 8): the reaction of 1a (1.0 mmol) and NaH (1.8 equiv.) in DCM/DMF (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3 mL) under ambient conditions gave the desired product 2a with an excellent yield of 98%.
5, 3 mL) under ambient conditions gave the desired product 2a with an excellent yield of 98%.
Under the optimized conditions, we explored the scope of the NaH-mediated reaction of DCM with various 2-oxazolidinones 1, and the results are shown in Table 2. It was found that the reactions of 2-oxazolidinones (including 2-oxazolidinone 1f, 4-substituted 2-oxazolidinones 1a–e, and benzoxazolidinone 1g) proceeded smoothly to afford the desired products in excellent yields. The reactivity of six-numbered cyclic carbamates, such as 1,3-oxazinan-2-one 1h and 1,4-dihydro-2H-benzo[d][1,3]oxazin-2-one 1i, was similar to oxazolidinones 1a-g and gave the corresponding methylene-bridged products 2h and 2i in 89% and 84% yields, respectively. Note that the reactions could not proceed when acyclic carbamates 1j–l were employed.
| No. | Substance | Time (h) | Product | Yieldb (%) |
|---|---|---|---|---|
a All the reactions were carried out with 1 (1 mmol) and NaH (1.8 equiv.) in DCM/solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3 mL) at ambient temperature (25–30 °C).b Isolated yield of 2.c The desired product was not observed. 5, 3 mL) at ambient temperature (25–30 °C).b Isolated yield of 2.c The desired product was not observed. |
||||
| 1 | 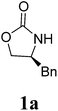 |
9 | 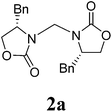 |
98 |
| 2 | 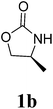 |
16 | 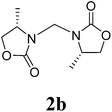 |
89 |
| 3 | 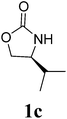 |
13 | 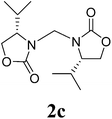 |
95 |
| 4 | 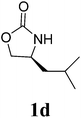 |
17 | 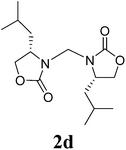 |
91 |
| 5 | 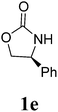 |
13 | 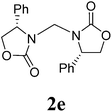 |
90 |
| 6 | 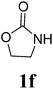 |
16 | 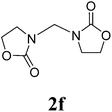 |
83 |
| 7 | 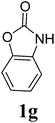 |
16 | 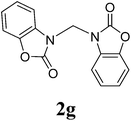 |
87 |
| 8 | 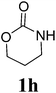 |
19 | 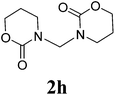 |
89 |
| 9 | 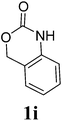 |
19 | 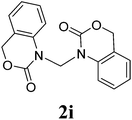 |
84 |
| 10 | 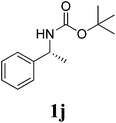 |
24 | — | —c |
| 11 | 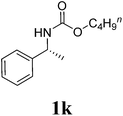 |
24 | — | —c |
| 12 | 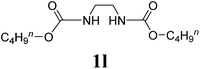 |
24 | — | —c |
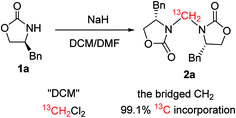
Considering that both the DCM and DMF can act as single carbon source,2–12,24 a labeling experiment was performed (Scheme 1) to elucidate the carbon source of the bridged methylene. The result of the reaction with 13C-labeled DCM indicated that the bridged methylene of compound 2 originated entirely from CH2Cl2 (99.1% incorporation) instead of DMF and other unknown substances from the NaH reagent (60% dispersion in mineral oil).25
Because DCM serves as a reagent in the transformation of 1 to 2, we propose that the ability of DMF, a strong polar aprotic solvent, in playing important role in the transformation is a result of the polar solvent's solvating certain reactant intermediate species, thereby decreasing the intermediate energy relative to the starting material. A series of control experiments supported this proposal to some extent (Table 3). It was found that the reactions proceeded smoothly to afford the desired product 2a in good to excellent yields, when different polar solvents, including strong polar DMSO, and other amides, such as N,N-diethylformamide, N,N-dimethylbutyramide, N,N-diethylbutyramide, and N,N-diethylbenzamide were employed. In DMSO (Table 3, entry 1), 1a reacted with DCM in the presence of NaH at ambient conditions to give 2a in a yield of 95%, although it took a longer time than in DMF (Table 2, entry 1).
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
a Unless otherwise indicated, all the reactions were carried out with 1a (1 mmol) and NaH (1.8 equiv.) in DCM/solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3 mL) at ambient temperature (25–30 °C).b Isolated yield of 2a.c Yield is determined by LC-MS.d The reaction was performed at 45 °C. 5, 3 mL) at ambient temperature (25–30 °C).b Isolated yield of 2a.c Yield is determined by LC-MS.d The reaction was performed at 45 °C. |
|||
| 1 | DMSO | 24 | 95 |
| 2 | 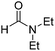 |
11 | 80 |
| 3 | 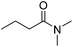 |
24 | 81c |
| 4 | 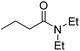 |
10 | 85c |
| 5 | 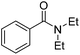 |
8d | 68c |
To our delight, a reactant intermediate was detected by LC-MS in small amounts in the control experiment, in which N,N-diethylformamide was employed as a solvent (Table 3, entry 2). The presence of a 3-methylene-4-benzyl-2-oxazolidinonium nucleus was strongly indicated by observing the peak at m/z 190. After enrichment and purification, the intermediate was characterized as 3-chloromethyl-4-benzyl-2-oxazolidinone A (Scheme 2) by 1H NMR and 13C NMR. 1H NMR in CDCl3 of the intermediate showed a doublet at 4.86 ppm and 4.70 ppm, and 13C NMR showed a resonance at 56.1 ppm. Both the sets of NMR data are consistent with A as the major species in the reaction solvent. Added 2-oxazolidinone 1a to pure intermediate A which was obtained from the experiment (Table 3, entry 2), under standard condition showed that intermediate A fast loss the resonances at 4.86 ppm and 4.70 ppm with the formation of a broad singlet at 4.98 ppm in the 1H NMR. This result indicated that the formation step of aminal 2 from the reaction intermediates is rapid.
On the basis of the abovementioned results and literature,26 a plausible mechanism for the reaction is illustrated in Scheme 2. Although the detailed mechanism of the present reaction is still not very clear, the following speculation is reasonable. Cyclic carbamate 1 undergoes N-alkylation with dichloromethane in the presence of NaH to chloromethyl analogue A. Then, the transient acyliminium intermediate B (from the balance between species A and B) rapidly undergoes a fast reaction with another nucleophile 1 present in the reaction to form the product 2. The condensation of DCM with 1 is the rate-determining step. The strong polar solvent DMF probably plays an important role by stabilizing the formation of the acyliminium ion intermediate B, which makes DCM more available to react with the nucleophile 1. However, why the reaction of acyclic carbamate does not work is not yet clear.
In conclusion, we have demonstrated a highly efficient and facile NaH-catalyzed condensation of DCM with cyclic carbamates to form corresponding aminal derivatives in a mixture solvent of DCM and DMF under ambient temperature. Isotopic labeling experiments indicated that the bridged methylene group was derived from DCM instead of DMF and other unknown substances in NaH reagent (60% dispersion in mineral oil). This protocol not only extends the application of DCM in organic synthesis but also provides an alternative method for preparing methylene-bridged bis(oxazolidin-2-one) derivatives. However, it is currently limited in scope to acyclic carbamates. Further studies to discover synthetic applications are ongoing.
Experimental section
General information
All the reagents were obtained from commercial sources and used without further purification. NaH (60% dispersion in mineral oil) was purchased from Aladdin Industrial Corporation. The reactions were monitored by TLC on silica gel 60 F254. Column chromatography was performed on silica gel 60 (200–300 mesh). The 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz, respectively. Chemical shifts were reported in ppm using tetramethylsilane (TMS) as an internal standard in CDCl3 solutions, and coupling constants (J) are given in Hz. High-resolution mass spectra were measured on a MicroTOF mass spectrometer.General procedure for the preparation of compound 2
A mixture of carbamate 1 (1.0 mmol) and NaH (1.8 equiv., 60% dispersion in mineral oil) in DMF/DCM (5/1, 3 mL) was stirred at room temperature for an appropriate duration, and the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was quenched with brine. The organic layer was separated and evaporated, the residue was added to H2O (10 mL) and washed with CH2Cl2, and the combined organics were then concentrated in vacuo. The crude product was purified by silica-gel column chromatography and characterized.3,3′-Bis((S)-4-benzyl-2-oxazolidinon-3-yl)methane (2a)
1H NMR (CDCl3, 400 MHz) δ 7.31–7.19 (m, 10H), 4.99 (s, 2H), 4.20–4.08 (m, 6H), 3.56–3.55 (d, J = 3.2 Hz, 2H), 2.67–2.61 (dd, J = 9.6 Hz, 2H). 13C{1H} NMR (CDCl3, 100 MHz) δ 158.7, 135.0, 129.2, 129.0, 127.2, 67.7, 55.3, 48.4, 37.8. HRMS (ESI), m/z calcd. for C21H22N2NaO4 ([M + Na]+) 389.1472, found: 389.1474.3,3′-Bis((S)-4-methyl-2-oxazolidinon-3-yl)methane (2b)
1H NMR (CDCl3, 400 MHz) δ 4.78 (s, 2H), 4.35 (t, J = 8.0 Hz, 2H), 3.94–3.84 (m, 4H), 1.39 (d, J = 6.0 Hz, 6H). 13C{1H} NMR (CDCl3, 100 MHz) δ 158.4, 69.4, 50.0, 47.7, 17.7. HRMS (ESI), m/z calcd. for C9H14N2NaO4 ([M + Na]+) 237.0846, found: 237.0842.3,3′-Bis((S)-4-isopropyl-2-oxazolidinon-3-yl)methane (2c)
1H NMR (CDCl3, 400 MHz) δ 4.77 (s, 2H), 4.26 (t, J = 9.2 Hz, 2H), 4.11 (dd, J = 6.0 Hz, J = 9.2 Hz, 2H), 3.85–3.81 (m, 2H), 2.46–2.39 (m, 2H), 0.89 (d, J = 7.2 Hz, 6H), 0.85 (d, J = 7.2 Hz, 6H). 13C{1H} NMR (CDCl3, 100 MHz) δ 158.9, 63.3, 57.8, 48.1, 26.4, 17.8, 13.9. HRMS (ESI), m/z calcd. for C13H22N2NaO4 ([M + Na]+) 293.1472, found: 293.1475.3,3′-Bis((S)-4-isobutyl-2-oxazolidinon-3-yl)methane (2d)
1H NMR (CDCl3, 400 MHz) δ 4.77 (s, 2H), 4.44 (t, J = 8.4 Hz, 2H), 3.98 (q, J = 8.4 Hz, 2H), 3.88–3.81 (m, 2H), 2.03–1.96 (m, 2H), 1.58–1.57 (m, 2H), 1.40–1.33 (m, 2H), 0.97–0.92 (m, 12H). 13C{1H} NMR (CDCl3, 100 MHz) δ 158.4, 68.5, 52.6, 47.9, 40.5, 24.4, 23.7, 21.5. HRMS (ESI), m/z calcd. for C15H26N2NaO4 ([M + Na]+) 321.1785, found: 321.1789.3,3′-Bis((S)-4-phenzyl-2-oxazolidinon-3-yl)methane (2e)
1H NMR (CDCl3, 400 MHz) δ 7.33–7.19 (m, 10H), 4.99 (s, 2H), 4.11–4.02 (m, 4H), 3.56–3.53 (m, 2H). 13C{1H} NMR (CDCl3, 100 MHz) δ 158.8, 135.0, 129.3, 129.0, 127.3, 67.7, 55.4, 48.4. HRMS (ESI), m/z calcd. for C19H18N2NaO4 ([M + Na]+) 361.1159, found: 361.1160.3,3′-Bis(2-oxazolidinon-3-yl)methane (2f)
1H NMR (CDCl3, 400 MHz) δ 4.77 (s, 2H), 4.35 (t, J = 8.0 Hz, 2H), 3.68 (t, J = 8.0 Hz, 2H). 13C{1H} NMR (CDCl3, 100 MHz) δ 158.6, 62.3, 53.5, 44.1. HRMS (ESI), m/z calcd. for C7H10N2NaO4 ([M + Na]+) 209.0533, found: 209.0530.3,3′-Bis(benz-2-oxazolidinon-3-yl)methane (2g)
1H NMR (CDCl3, 400 MHz) δ 7.57 (s, 2H), 7.26 (s, 2H), 7.21 (s, 4H), 5.89 (s, 2H). 13C{1H} NMR (CDCl3, 100 MHz) δ 154.4, 142.4, 128.9, 124.8, 123.9, 110.3, 110.2, 48.5, 29.7. HRMS (ESI), m/z calcd. for C15H10N2NaO4 ([M + Na]+) 305.0533, found: 305.0534.3,3′-bis(1,3-oxazinan-2-on-3-yl)methane (2h)
1H NMR (CDCl3, 400 MHz) δ 4.90 (s, 2H), 4.20 (t, J = 5.2 Hz, 4H), 3.44 (t, J = 6.0 Hz, 4H), 1.98–1.92 (m, 4H). 13C{1H} NMR (CDCl3, 100 MHz) δ 154.3, 67.0, 62.1, 45.1, 22.1. HRMS (ESI), m/z calcd. for C9H14N2NaO4 ([M + Na]+) 237.0846, found: 237.0848.3,3′-Bis(1,4-dihydro-2H-benz[d][1,3]-oxazinan-2-on-3-yl)methane (2i)
1H NMR (CDCl3, 400 MHz) δ 7.40 (s, 2H), 7.32 (s, 2H), 7.11 (s, 4H), 6.36 (s, 2H), 4.97 (s, 4H). 13C{1H} NMR (CDCl3, 100 MHz) δ 153.5, 134.9, 129.7, 124.9, 124.1, 121.6, 114.2, 67.2, 51.2. HRMS (ESI), m/z calcd. for C17H14N2NaO4 ([M + Na]+) 333.0846, found: 333.0841.Acknowledgements
We thank the NSFC (21002051, 21172056, 21272057 and 21372065), PCSIRT (IRT1061), China Postdoctoral Science Foundation funded project (2012M521397, 2013T60701 and 2013M530339), and Key Project of Henan Educational Committee (13A150546) for the financial support for this research.References
- (a) M. Heckenroth, A. Neels, M. G. Garnier, P. Aebi, A. W. Ehlers and M. Albrecht, Chem. – Eur. J., 2009, 15, 9375 CrossRef CAS PubMed; (b) A. Otero, J. Fernández-Baeza, A. Antiñolo, J. Tejeda, A. Lara-Sánchez, L. Sánchez-Barba, M. T. Expósito and A. M. Rodríguez, Dalton Trans., 2003, 1614 RSC; (c) A. Beck, B. Weibert and N. Burzlaff, Eur. J. Inorg. Chem., 2001, 521 CrossRef CAS; (d) I. Hegelmann, A. Beck, C. Eichhorn, B. Weibert and N. Burzlaff, Eur. J. Inorg. Chem., 2003, 339 CrossRef CAS; (e) A. Otero, F. Carrillo-Hermosilla, P. Terreros, T. Expósito, S. Rojas, J. Fernández-Baeza, A. Antiñolo and I. López-Solera, Eur. J. Inorg. Chem., 2003, 3233 CrossRef CAS; (f) A. Otero, J. Fernández-Baeza, J. Tejeda, A. Antiñolo, F. Carrillo-Hermosilla, E. Díez-Barra, A. Lara-Sánchez and M. Fernández-López, J. Chem. Soc., Dalton Trans., 2000, 2367 RSC; (g) D. L. Reger, J. R. Gardinier, P. J. Pellechia, M. D. Smith and K. J. Brown, Inorg. Chem., 2003, 42, 7635 CrossRef CAS PubMed; (h) F. DeRosa, X. Bu and P. C. Ford, Inorg. Chem., 2003, 42, 4171 CrossRef CAS PubMed; (i) S. Tardito, I. Bassanetti, C. Bignardi, L. Elviri, M. Tegoni, C. Mucchino, O. Bussolati, R. Franchi-Gazzola and L. Marchio, J. Am. Chem. Soc., 2011, 133, 6235 CrossRef CAS PubMed; (j) S. H. Oakley, M. P. Coles and P. B. Hitchcock, Inorg. Chem., 2004, 43, 7564 CrossRef CAS PubMed; (k) K. Fujisawa, R. Kanda, Y. Miyashita and K. Okamoto, Polyhedron, 2008, 27, 1432 CrossRef CAS PubMed.
- A. S. McCall, H. Wang, J. M. Desper and S. Kraft, J. Am. Chem. Soc., 2011, 133, 1832 CrossRef CAS PubMed.
- S. H. Hansen and L. Nordholm, J. Chromatogr. A, 1981, 204, 97 CrossRef CAS.
- A. H. Beckett and H. M. Ali, J. Chromatogr. A, 1979, 177, 255 CrossRef CAS.
- D. Wright and C. Wulff, J. Org. Chem., 1970, 35, 4252 CrossRef.
- J. E. Mills, C. A. Maryanoff, D. F. McComsey, R. C. Stanzione and L. Scott, J. Org. Chem., 1987, 52, 1857 CrossRef CAS.
- E. Díez-Barra, A. de la Hoz, A. Sánchez-migallón and J. Tejeda, Heterocycles, 1992, 34, 1365 CrossRef.
- J. E. Mills, C. A. Maryanoff, D. F. McComsey, R. C. Stanzione and L. Scott, J. Org. Chem., 1987, 52, 1857 CrossRef CAS.
- U. Burger and F. Dreier, Tetrahedron, 1983, 39, 2065 CrossRef CAS.
- (a) L. D. Field, B. A. Messerle, M. Rehr, L. P. Soler and T. W. Hambley, Organometallics, 2003, 22, 2387 CrossRef CAS; (b) S. Tardito, I. Bassanetti, C. Bignardi, L. Elviri, M. Tegoni, C. Mucchino, O. Bussolati, R. Franchi-Gazzola and L. Marchiò, J. Am. Chem. Soc., 2011, 133, 6235 CrossRef CAS PubMed.
- A. B. Rudine, M. G. Walter and C. C. Wamser, J. Org. Chem., 2010, 75, 4292 CrossRef CAS PubMed.
- (a) A. Hassner, M. Stern, H. E. Gottlieb and F. Frolow, J. Org. Chem., 1990, 55, 2304 CrossRef CAS; (b) L. Avila, J. Elguero, S. Juliá and J. M. del Mazo, Heterocycles, 1983, 20, 1787 CrossRef CAS.
- (a) D. A. Evans, J. Bartroli and T. L. Shih, J. Am. Chem. Soc., 1981, 103, 2127 CrossRef CAS; (b) D. A. Evans, R. L. Dow, T. L. Shih, J. M. Takacs and R. Zahler, J. Am. Chem. Soc., 1990, 112, 5290 CrossRef CAS; (c) R. Barth and W. R. Roush, Org. Lett., 2010, 12, 2342 CrossRef CAS PubMed.
- (a) J. Kerherve, C. Botuha and J. Dubois, Org. Biomol. Chem., 2009, 7, 2214 RSC; (b) S. S. Koch and A. R. Chamberlin, J. Org. Chem., 1993, 58, 2725 CrossRef CAS; (c) D. A. Evans, F. Urpi, T. C. Somers, J. S. Clark and M. T. Bilodeau, J. Am. Chem. Soc., 1990, 112, 8215 CrossRef CAS.
- D. A. Evans, T. C. Britton, J. A. Ellman and R. L. Dorow, J. Am. Chem. Soc., 1990, 112, 4011 CrossRef CAS.
- U. Schmidt and B. Riedl, J. Chem. Soc., Chem. Commun., 1992, 1186 RSC.
- G. K. Friestard and A. Ji, Org. Lett., 2008, 10, 2311 CrossRef PubMed.
- (a) R. Hayashi, J. B. Feltenberger and R. Hsung, Org. Lett., 2010, 12, 1152 CrossRef CAS PubMed; (b) D. A. Evans, K. T. Chapman and J. Bisaha, J. Am. Chem. Soc., 1988, 110, 1238 CrossRef CAS.
- (a) J. P. John, J. Jost and A. V. Novikov, J. Org. Chem., 2009, 74, 6083 CrossRef CAS PubMed; (b) D. A. Evans, A. M. Ratz, B. E. Huff and G. S. Sheppard, J. Am. Chem. Soc., 1995, 117, 3448 CrossRef CAS; (c) D. E. Cane, W. Tan and W. R. Ott, J. Am. Chem. Soc., 1993, 115, 527 CrossRef CAS; (d) J. Montgomery, G. M. Wieber and L. S. Hegedus, J. Am. Chem. Soc., 1990, 112, 6255 CrossRef CAS.
- (a) R. K. Mishra, C. M. Coates, K. D. Revell and E. Turos, Org. Lett., 2007, 9, 575 CrossRef CAS PubMed; (b) C. M. Perry and B. Jarvis, Drugs, 2002, 61, 525 CrossRef PubMed; (c) S. J. Brickner, D. K. Hutchinson, M. R. Barbachyn, D. A. Ulanowicz, S. A. Gramon, K. C. Grega, S. K. Hendges, D. S. Toops, C. W. Ford and G. E. Zurenko, J. Med. Chem., 1996, 39, 673 CrossRef CAS PubMed; (d) T. K. Jones, R. A. Reamer, R. Desmond and S. G. Mills, J. Am. Chem. Soc., 1990, 112, 2998 CrossRef CAS; (e) J. Wouters, Curr. Med. Chem., 1998, 5, 137 CAS.
- R. F. J. Ingleby, GB Patent 887,595, 1962, Chem. Abstr. 1962, 56, 12904g.
- H. Zinner, H. Herbig and H. Wigert, Chem. Ber., 1956, 89, 2131 CrossRef CAS.
- C. Gaul, K. Schärer and D. Seebach, J. Org. Chem., 2001, 66, 3059 CrossRef CAS PubMed.
- (a) S. Lou, D. Xu, D. Shen, Y. Wang, Y. Liu and Z. Xu, Chem. Commun., 2012, 48, 11993 RSC; (b) Y. Lv, Y. Li, T. Xiong, W. Pu, H. Zhang, K. Sun, Q. Liu and Q. Zhang, Chem. Commun., 2013, 49, 6439 RSC.
- Carbon resonances were integrated and standardized to three different positions [C (159.0 ppm), C (135.1 ppm), C (127.4 ppm)]. In each case, the ratio of the integrated signal from the labeled sample to that from the unlabeled product was determined, and then the three sets were averaged. This quotient was multiplied by the natural abundance of 13C (1.1%) to give the % 13C incorporation at each carbon position of 13C-2a (see the ESI†). For the calculation method, also see: K. E. Roege and W. L. Kelly, Org. Lett., 2009, 11, 297 CrossRef CAS PubMed.
- (a) J. E. Mills, C. A. Maryanoff, D. F. McComsey, R. C. Stanzione and L. Scott, J. Org. Chem., 1987, 52, 1857 CrossRef CAS; (b) H. Federsel, E. Konberg, L. Lilljequist and B. Swahn, J. Org. Chem., 1990, 55, 2254 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC [997065]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra03248a |
| This journal is © The Royal Society of Chemistry 2014 |