A general method for mass and template-free production of hierarchical metal oxide spheres at room-temperature†
Chao Wanga,
Mingwei Zhu*a,
Hong Liub,
Yushuang Cuia and
Yanfeng Chena
aNational Laboratory of Solid State Microstructures & Department of Materials Science and Engineering, Nanjing University, Nanjing 210093, China. E-mail: mwzhu@nju.edu.cn; Fax: +86 2583595535; Tel: +86 2583594317
bState Key Laboratory of Crystal Materials, Shandong University, Jinan 250100, China. E-mail: hongliu@sdu.edu.cn
First published on 23rd May 2014
Abstract
Hierarchical metal oxide spheres (HMOS) have various applications that stem from their special architectures. Here a novel template-free method is developed for mass fabrication of HMOS at room temperature. It is only composed of the facile processes of PEG modification and self-assembly (PMSA). Its powerful fabrication capability is demonstrated by fabricating HMOS of TiO2, Fe2O3, ZrO2 and their mixtures with high quality. The related mechanism is investigated and the peculiar PEG configuration on the surface of the nanoparticle is believed to be the origin of the HMOS formation. Several possible applications of HOMS are also exhibited, such as the superhydrophobic properties, the enhanced light scattering ability and the improved solar cell performance. Considering the unique fabrication abilities and industrial mass production potentials, this method may be used as a powerful tool for the synthesis of HMOS with extended applications.
1. Introduction
Morphology modification of materials at the nanoscale is an important aspect for materials design and further development of their applications. For hierarchical architectures, which have dual sizes on the primary nanoscale and the secondary microscale in the same structure,1 some size related properties will arise, such as the enhanced light response and increased mass/charge exchange efficiency.2–4 Benefiting from the unique structures having both high specific surface area and large pore size,5 they have potential applications in areas of environmental treatments,6–9 solar cells,2,5,10,11 energy storage,3,12 gas/liquid sensing,4 etc.Among these hierarchical structures, hierarchical metal oxide spheres (HMOS) are especially useful and therefore a lot of efforts have been focused on their preparations. The most common route for synthesis of hierarchical architectures is template based methods.13 As a typical fabrication procedure, rigid (colloid spheres, rigid particles, etc.) or soft (liquid droplets, micelles, etc.) materials are used as templates, then they are coated with target materials and finally the hierarchical structures are acquired after removal of the templates. Many hierarchical metal oxide spheres (HMOS) have been synthesized with various materials using this method, such as TiO2,14 SiO2,15–18 C,19 ZnO,20 and Fe2O3.21,22 Template based method is considered as the most promising tool for synthesis of hierarchical architectures because they can efficiently control both the morphology and the microstructure.3,12 However, the template materials are often high cost and the synthetic procedures are complex and time consuming. Hence, template free methods are rapidly developed for synthesis HMOS of CuO,23,24 α-FeOOH,25 TiO2,26–31 ZnS,32 MnS,33 Fe2O3,34 MnO2,35 SnO2,36 Li4Ti5O2,37 SrTiO3,38 NiO,39 ZnO,10,11,40 etc. But their synthesis mainly relies on the hydro/solvothermal processes with high temperature or pressure synthesis conditions, which the hydro/solvothermal methods are difficult to be scaled up for mass production. Hence, low temperature synthesis methods are pursued for synthesis HMOS in much milder conditions.41 Despite these excellent efforts, the synthesis of HMOS is still a challenging field and the acquisition of HMOS is laborious. In view of practical applications, it is highly desirable to develop efficient and cost-effective methods for mass production of HMOS with powerful fabrication capabilities.
In this paper, we introduce a general and facile method for production of HMOS with kilograms fabrication potential. This template-free method mainly contains simple processes of PEG (polyethyleneglycol) modification followed by self-assembling (PMSA). However, HMOS of many materials, or even materials with designed multiple components, can be facilely acquired by this commercial available method. As demonstrations, we have fabricated HMOS with single component of TiO2, Fe2O3, ZrO2, two-components of TiO2–Fe2O3, and multi-components of TiO2–Fe2O3–ZrO2 to show the capability and universality of this method for fabrication of different HMOS.
2. Experimental
Preparation of HMOS
As a typical fabrication procedure, metal oxide nanoparticles were mixed with PEG (HO–[CH2–CH2–O]n–CH2–CH2–OH) in ball milling jars and grinded for 12 hours. Then the powders of 4.0 grams in weight were dispersed in solution containing 7.0 ml deionized water and 4 drops of nitric acid. After fully dispersion, the paste was coated on the surface of substrate by automatic film applicator coater (ZEHNTNER ZAA 2300) with controllable thicknesses. Then the film was dried and annealed at 450 °C for 30 min to remove the organics and other ingredients in the precursors.Fabrication of dye-sensitized solar cells (DSSC)
The electrodes were prepared by coating the TiO2 pastes on the surface of FTO glass plates by automatic film applicator coater with controllable thickness. After being dried in temperature humidity chamber, the thin film was annealed at 450 °C for 30 minutes. The obtained films were absorbed with N719 dye by the vacuum assistant dye adsorption processes. The counter Pt-electrodes was prepared by sputtering Pt catalyst about 3 nm in thickness on the FTO glass. The dye-adsorbed TiO2 electrode and Pt-counter electrode were assembled into a sandwich type cell and sealed with a hot-melt film (ionomer Surlyn 1702, Dupont) of about 60 μm in thickness. Finally, the electrolyte (0.1 M LiI, 0.05 M I2, 0.5 M 4-tert-butylpyridine in acetonitrile) was filled in and the hole was sealed using the Surlyn 1702 hot-melt film and a cover glass.Measurements and characterizations
The surface and cross-section morphologies of HMOS were characterized by a scanning electron microscope (SEM, FEI Quanta200 and ZEISS ULTRA 55). The details of the hierarchical TiO2 spheres were observed by transmission electron microscope (TEM, FEI TECNA1F20 200 kV). The Infrared and diffused reflection spectra were tested by Perkin Elmer spectrophotometer (Spectrum GX) through KBr (Alfa Aesar) wafer method. The static and dynamic contact angles were measured by Contact Angle Meter (OCA30). The Pt counter electrodes were spattered by precision etching coating system (Model 682, Gatan). Photocurrent–voltage characteristics of solar cells were measured using a voltage–current source monitor (Orzel Sol 3A, Newport) under illumination by a Newport solar simulator (AM 1.5, 100 mW cm−2) with a scan rate of 10 mV s−1.3. Results and discussion
The PMSA method is schematically illustrated in Fig. 1. Its fabrication procedure mainly contains three steps. Firstly, the metal oxide powder is mixed with PEG (polyethyleneglycol) by ball milling to realize their close contact (step 1). Then the resulted powders are dispersed in water and stirred to provide the condition for nanoparticles self-assembly (step 2). Finally, the resulted HMOS are coated on substrate and the PEG can be removed by sintering (step 3).Fig. 2 shows the SEM and TEM images of the obtained TiO2, Fe2O3 and ZrO2 hierarchical spheres. Both the surface (Fig. 2a) and cross-section (Fig. 2b) SEM images indicate that the TiO2 film is completely composed of spherical hierarchical spheres about 1 μm in diameter, morphologically very different form the original TiO2 nanoparticles (Fig. S1†). Each hierarchical sphere is constructed by densely stacked TiO2 nanoparticles of about 30 nm in diameter (Fig. 2c and d). The pore size is about 20 nm (Fig. S2†) and the surface area of TiO2 HMOS is 50.60 m2 g−1. Also, the XRD results (Fig. S3†) indicate that the PEG removal process by sintering does not change the crystalline structure and inherent properties of metal oxide. Similar structural characters are found in HMOS of ZrO2 and Fe2O3 shown in Fig. 2e and f with diameters about 200 nm and 0.8 μm, respectively. All these results demonstrate that the PMSA method is an effective approach for fabricating hierarchical metal oxide spheres.
The most important advantage of our method is that it is facile whereas powerful in mass production of HOMS, which is important for their practical uses or application area extensions. This advantage stems from the unique characteristics of the method from raw materials to fabrication procedure. The relatively low-price commercial metal oxide nanoparticles are adopted as the starting raw materials. Low-cost and efficient ball milling mixing process, a way commonly used in laboratories and factories, acts as the main fabrication step. All these characteristics endow the PMSA method with the potentials for industrialized mass production of HMOS. In our demonstrated experiments, the amount of productivity is limited by the capacity of the ball mill jars we used. For example, using jar mill of 50 ml, the weights of HMOS of TiO2, Fe2O3 and ZrO2 are about 21.33 g, 17.37 g and 9.37 g, respectively (Fig. S4†). However, We believe that this method can be facilely scaled up and HMOS of kilograms in weight may be efficiently obtained by using much bigger or more ball milling jars.
Another advantage of the PMSA method is that it may create novel HMOS composed of mixtures of different metal oxide nanoparticles. Such mixed poly-nanocrystals may display profoundly improved properties than any of the pure nanocrystal,14,42–44 but their synthesis is considered to be a challenge. Here we use TiO2–Fe2O3 and TiO2–Fe2O3–ZrO2 for demonstrations and the results are shown in Fig. S5.† These images clearly show that the hierarchical architectures can also form for two-components of TiO2–Fe2O3 and multi-components of TiO2–Fe2O3–ZrO2. The EDX measurements (Fig. S5†) show that the nanoparticles with different materials are mixed together, and each single sphere contains designed kinds of nanoparticles. Such results indicate that different metal oxide nanoparticles jointly participate in the construction of the structures. We believe that the hierarchical spheres can also be fabricated using with various prescriptions for satisfying different application requirements.
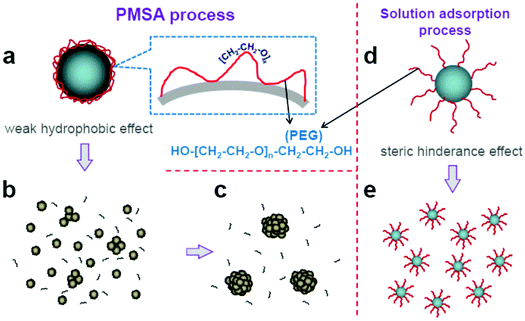
The formation of HMOS is surely the result of the minimization of Gibbs free energy in the systerm.14,32,36 However, we would like to investigate the details in our system. PEG (HO–[CH2–CH2–O]n–CH2–CH2–OH) is often used as surfactant for avoiding aggregation of nanoparticles base on the stereo-hindrance effect. On the contrary, they are the origin for the appropinquity between adjacent nanoparticles. Also, the experiments show that the PEG and dry grinding treatment are essential for the HMOS formation and hierarchical structures are not observed if only one of them was used (Fig. S6 and S7†). Hence we believe that such difference origins from the different configurations that the PEG molecules adsorbed on the particle surface. For the commonly used solution adsorption at high surface coverage levels, the “brush” configuration,45 with one terminal of PEG molecule fixed on the particle surface and the other part extends to the outer solution, is the main manner (Fig. 3d). However, for PEG molecule treated by dry grinding process, the “lying” configuration (Fig. 3a), with PEG molecule lies on the particle surface via a number of linkage sites through hydrogen bonding,46,47 is believed to be the main mode (Fig. S8,† infrared transmittance spectra). Such configuration can realize stronger interaction between PEG molecule chain and the nanoparticle. More importantly, the “lying” configuration makes the –OH terminals in PEG molecule fixed on the particle surface and exposes the [–CH2–CH2–O]x forming unit outside. This is the key point and it makes the PEG linked nanoparticles display the properties of [–CH2–CH2–O]x segment during their interaction or interaction with solvent. Consequently, the aggregation of PEG-linked nanoparticles should be very similar to that of PEO (poly(ethylene oxide)) in aqueous solution with segment–segment and segment–solvent interactions. Although the origin of their aggregation is still debated, the hydrophobic interactions48,49 (the –CH2–CH2– groups repel water) are believed to serve as the main driving force of the aggregation for PEG-linked nanoparticles in our experiments.
Accordingly, a possible mechanism is proposed to explain the formation of HMOS shown in Fig. 3. Firstly, PEG molecule is bonded on the surface of metal oxide nanoparticle via a number of linkage sites (Fig. 3a). Then, many tiny “clusters” form after the PEG linked nanoparticles dispersed in water (Fig. 3b). Subsequently, these clusters serve as “nuclei” and slowly grow up by continuous accepting individual nanoparticles from their surrounding. Finally, hierarchical spheres with certain sizes come into being after all the residual individual nanoparticles used up (Fig. 3c).
Based on the above aggregation formation mechanism, the molecule weight (MW) of PEG, which determines the molecule chain length, may affect the formation of hierarchical architectures by changing their surface hydrophobic properties. The shorter the molecule chain, the more negative contribution of the –OH end groups to the hydrophobic interactions of the –CH2–CH2– groups. Hence, short chain of PEG should be disadvantageous for the HMOS formation. The experimental results are shown in Fig. S9† using TiO2 as examples with various molecular weights. Evidently, the hierarchical characteristics weaken gradually with MW decreasing from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 10
000, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 4000 and finally diminish completely for 2000. Also, surface coverage level can affect the hydrophobic interactions and thereby affect the particle aggregation. However, the mass ratios of TiO2 to PEG show less important influence on the configuration of the spherical hierarchical structures as shown in Fig. S10.†
000, 4000 and finally diminish completely for 2000. Also, surface coverage level can affect the hydrophobic interactions and thereby affect the particle aggregation. However, the mass ratios of TiO2 to PEG show less important influence on the configuration of the spherical hierarchical structures as shown in Fig. S10.†
The uniformity of the resulted aggregates may be interpreted by LaMer model,50 which is often used for explaining the growth mechanism of mono-dispersed submicron particles (Fig. S11†). At the starting point, the adjacent PEG linked nanoparticles self-assemble and form many “clusters” within a very short time. This process accompanies by a sharp decrease of the “particle number density” in solution. After that, no new “clusters” are generated because of the enlarged particle distance. The already formed “clusters” gradually grow up and produce uniform particle aggregates.
The resulted HMOS can be facile used without further treatments or requisition for critical construction conditions, equipments, or substrates. They can even be coated on a curved surface of an optical fiber compactly and uniformly (Fig. 4).
Hence, they can be facile used in many areas. Because TiO2 is the most intensively studied metal oxide nanomaterials for its wide applications,51 it is selected to develop the application of HMOS. From the SEM observation in Fig. 2, it is noted that the surface configuration of the HMOS film contains both nano and micron structures, which are very similar to the surface structure of lotus leaf.52 Therefore, such configurations can be used to bionic the hydrophobic effect of lotus leaf.53 The as prepared hierarchical TiO2 spheres are superhydrophilic. However, the HMOS film becomes super-hydrophobic after surface fluoridated (Fig. 5). The measured static contact angle of the TiO2 film after fluorinating is about 162.6° and dynamic contact angle is about 4°. These values show excellent super-hydrophobic performance, which may be used in self-cleaning and photochemical catalysis.
Also, the HMOS have enhanced light scattering capability caused by the microsized architecture in the hierarchical spheres.54 Fig. 6a and b show the schematic and measured diffused reflection spectra for TiO2 films at different configurations, respectively. The P25 nanoparticle film shows the lowest light scattering efficiency, which is rational because their particle size is far smaller than the wavelengths of the incident light. For films prepared by using PEG-2000 and 4000, there is little difference in diffused reflection. With the PEG molecular weight increases from 4000 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the diffused reflection increases by almost 20% at all the visible light wavelengths. Such an increase is in correspondence with the structural evolution from the nanoparticles gradually to hierarchical spheres. Because the band gap of TiO2 is about 3.2 eV, they cannot absorb the visible light. The increased diffused reflection indicates the stronger light scattering in the film. Hence, hierarchical spheres can improve the light scattering ability proved by the fact that, the more contents of hierarchical spheres in TiO2 film, the stronger diffused reflection appears. The enhanced light scattering ability can certainly be used to enhance the light harvest of many photo-electron conversion related devices by increasing the probability of interaction between the photons and the light active materials.10,11 Here we studied the effects of hierarchical structures on the performances of dye-sensitized solar cells (DSSC). From the I–V curves (Fig. 6c) we can see that the short-current densities are enlarged apparently with the increasing of PEG molecule weights. An increase of about 140% for Jsc is observed comparing PEG-20
000, the diffused reflection increases by almost 20% at all the visible light wavelengths. Such an increase is in correspondence with the structural evolution from the nanoparticles gradually to hierarchical spheres. Because the band gap of TiO2 is about 3.2 eV, they cannot absorb the visible light. The increased diffused reflection indicates the stronger light scattering in the film. Hence, hierarchical spheres can improve the light scattering ability proved by the fact that, the more contents of hierarchical spheres in TiO2 film, the stronger diffused reflection appears. The enhanced light scattering ability can certainly be used to enhance the light harvest of many photo-electron conversion related devices by increasing the probability of interaction between the photons and the light active materials.10,11 Here we studied the effects of hierarchical structures on the performances of dye-sensitized solar cells (DSSC). From the I–V curves (Fig. 6c) we can see that the short-current densities are enlarged apparently with the increasing of PEG molecule weights. An increase of about 140% for Jsc is observed comparing PEG-20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 sample with the no PEG contrast sample. This trend also corresponds to the light scattering abilities shown in Fig. 6b. In correspondence, shown in Table 1, the electrode using TiO2 hierarchical spheres fabricated by PEG-20
000 sample with the no PEG contrast sample. This trend also corresponds to the light scattering abilities shown in Fig. 6b. In correspondence, shown in Table 1, the electrode using TiO2 hierarchical spheres fabricated by PEG-20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 gives the highest efficiency of 5.30%, which was dramatically higher than the 2.63% obtained for the contrast P25 nanocrystallites. These results indicate that the TiO2 hierarchical spheres can greatly improve the photovoltaic performance by enhancing light scattering and harvest. In fact, the enhanced transport of injected electrons and/or the facilitated diffusion of the electrolyte by the hierarchical architecture may also have some contributions.2 Moreover, it should be noted that the commercial TiO2 nanoparticles (Degussa P25) were chosen as the basic material for fabrication of DSSC and all the techniques for improving the efficiency were abandoned during the cell fabrication processes. Such designs may further enable the acquisition of reliable results about the effects of TiO2 hierarchical spheres on the DSSC performances.
000 gives the highest efficiency of 5.30%, which was dramatically higher than the 2.63% obtained for the contrast P25 nanocrystallites. These results indicate that the TiO2 hierarchical spheres can greatly improve the photovoltaic performance by enhancing light scattering and harvest. In fact, the enhanced transport of injected electrons and/or the facilitated diffusion of the electrolyte by the hierarchical architecture may also have some contributions.2 Moreover, it should be noted that the commercial TiO2 nanoparticles (Degussa P25) were chosen as the basic material for fabrication of DSSC and all the techniques for improving the efficiency were abandoned during the cell fabrication processes. Such designs may further enable the acquisition of reliable results about the effects of TiO2 hierarchical spheres on the DSSC performances.
| Sample | Thickness [μm] | Voca [V] | Jscb [mA cm−2] | Fill factor [%] | Efficiency [%] |
|---|---|---|---|---|---|
| a Open-circuit voltage.b Short-circuits current density. | |||||
PEG-20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.2 | 0.66 | 13.86 | 57.93 | 5.30 |
PEG-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.7 | 0.67 | 13.50 | 53.97 | 4.91 |
| PEG-4000 | 4.9 | 0.64 | 11.06 | 54.07 | 3.80 |
| PEG-2000 | 7.1 | 0.67 | 9.80 | 47.65 | 3.19 |
| PEG-NO | 6.5 | 0.70 | 5.77 | 64.60 | 2.63 |
4. Conclusion
In summary, we have developed a PMSA method for mass production of hierarchical metal oxide spheres (HMOS). HMOS with single component of TiO2, Fe2O3 or ZrO2, two-components of TiO2–Fe2O3, and multi-components of TiO2–Fe2O3–ZrO2 are fabricated to demonstrate the processes and capability of this method. The “lying” configuration of PEG molecule on the particle surface is the key point for the formation of HMOS. Due to the microsized particle diameters, HMOS of TiO2 show enhanced light scattering ability. When used as electrode in dye sensitized solar cells, a significant improvement of photon-to-current conversion efficiency is achieved compared with P25 nanoparticles. Because of the generality and industrial mass production potential, the PMSA method may enable the synthesis of various interesting HMOS and thereby develop important applications on many fields, such as photocatalysis, solar cells, sensing devices and optics.Acknowledgements
This work was jointly supported by the State Key Program for Basic Research of China (no. 2013CB632702 and 2012CB921503), National Natural Science Foundation of China (no. 11134006 and 51172105), the Nature Science Foundation of Jiangsu Province (no. BK2011574), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- T. P. Chou, Q. F. Zhang, G. E. Fryxell and G. Z. Cao, Adv. Mater., 2007, 19, 2588 CrossRef CAS.
- Q. F. Zhang and G. Z. Cao, J. Mater. Chem., 2011, 21, 6769 RSC.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604 CAS.
- J. H. Lee, Sens. Actuators, B, 2009, 140, 319 CrossRef CAS PubMed.
- Y. J. Kim, M. Lee, H. J. Kim, G. Lim, Y. S. Choi, N. G. Park, K. Kim and W. I. Lee, Adv. Mater., 2009, 21, 3668 CrossRef CAS.
- J. H. Pan, H. Q. Dou, Z. G. Xiong, C. Xu, J. Z. Ma and X. S. Zhao, J. Mater. Chem., 2010, 20, 4512 RSC.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., B, 2012, 13, 169 CrossRef CAS PubMed.
- T. Zhu, J. S. Chen and X. W. Lou, J. Phys. Chem. C, 2012, 116, 6873 CAS.
- Y. Q. Wang, G. Z. Cao, H. Q. Wang, W. P. Cai, C. H. Liang and L. D. Zhang, Nanotechnology, 2009, 20, 155604 CrossRef PubMed.
- Q. F. Zhang, T. P. Chou, B. Russo, S. A. Jenekhe and G. Z. Cao, Angew. Chem., 2008, 120, 2436 CrossRef.
- Q. F. Zhang, T. P. Chou, B. Russo, S. A. Jenekhe and G. Z. Cao, Adv. Funct. Mater., 2008, 18, 1654 CrossRef CAS.
- Z. Y. Wang, L. Zhou and X. W. Lou, Adv. Mater., 2012, 24, 1903 CrossRef CAS.
- W. Wei and Z. Z. Yang, Adv. Mater., 2008, 20, 2965 CrossRef CAS.
- X. J. Lv, F. Q. Huang, J. J. Wu, S. J. Ding and F. F. Xu, ACS Appl. Mater. Interfaces, 2011, 3, 566 Search PubMed.
- J. Wang, Q. Xiao, H. Zhou, P. Sun, Z. Yuan, B. Li, D. Ding, A. C. Shi and T. Chen, Adv. Mater., 2006, 18, 3284 CrossRef CAS.
- J. G. Wang, F. Li, H. J. X. Zhou, P. C. Sun, D. T. Ding and T. H. Chen, Chem. Mater., 2009, 21, 612 CrossRef CAS.
- J. Liu, S. Z. Qiao, S. B. Hartono and G. Q. Lu, Angew. Chem., 2010, 122, 5101 CrossRef.
- X. Wu, Y. Tian, Y. Cui, L. Wei, Q. Wang and Y. Chen, J. Phys. Chem. C, 2007, 111, 9704 CAS.
- Q. Li, R. Jiang, Y. Dou, Z. Wu, T. Huang, D. Feng, J. Yang, A. Yu and D. Zhao, Carbon, 2011, 49, 1248 CrossRef CAS PubMed.
- Y. Qiu, W. Chen, S. Yang, B. Zhang, X. X. Zhang, Y. C. Zhong and K. S. Wong, Cryst. Growth Des., 2010, 10, 177 CAS.
- B. Wang, J. S. Chen, H. B. Wu, Z. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146 CrossRef CAS PubMed.
- J. Yu, X. Yu, B. Huang, X. Zhang and Y. Dai, Cryst. Growth Des., 2009, 9, 1474 CAS.
- H. Zhang, Q. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766 CrossRef CAS.
- R. Liu, J. Yin, W. Du, F. Gao, Y. Fan and Q. Lu, Eur. J. Inorg. Chem., 2013, 1358 CrossRef CAS.
- B. Wang, H. Wu, L. Yu, R. Xu, T. T. Lim and X. W. Lou, Adv. Mater., 2012, 24, 1111 CrossRef CAS PubMed.
- W. G. Yang, J. M. Li, Y. L. Wang, F. Zhu, W. M. Shi, F. R. Wan and D. S. Xu, Chem. Commun., 2011, 47, 1809 RSC.
- Z. Zheng, B. B. Huang, X. Qin, X. Zhang and Y. Dai, Chem.–Eur. J., 2010, 16, 11266 CrossRef CAS PubMed.
- D. H. Chen, F. Z. Huang, Y. B. Cheng and R. A. Caruso, Adv. Mater., 2009, 21, 2206 CrossRef CAS.
- J. H. Pan, Z. Cai, Y. Yu and X. S. Zhao, J. Mater. Chem., 2011, 21, 11430 RSC.
- H. Zhang, H. Yu, Y. Han, P. Liu, S. Zhang, P. Wang, Y. B. Chen and H. Zhao, Nano Res., 2011, 4, 938 CrossRef CAS PubMed.
- L. S. Zhong, J. S. Hu, L. J. Wan and W. G. Song, Chem. Commun., 2008, 1184 RSC.
- Q. Zhao, Y. Xie, Z. Zhang and X. Bai, Cryst. Growth Des., 2007, 7, 153 CAS.
- Y. Cheng, Y. Wang, C. Jia and F. Bao, J. Phys. Chem. B, 2006, 110, 24399 CrossRef CAS PubMed.
- Z. Wu, K. Yu, S. Zhang and Y. Xie, J. Phys. Chem. C, 2008, 112, 11307 CAS.
- P. Yu, X. Zhang, D. Wang, L. Wang and Y. Ma, Cryst. Growth Des., 2009, 9, 528 CAS.
- Z. J. Miao, Y. Y. Wu, X. R. Zhang, Z. M. Liu, B. X. Han, K. L. Ding and G. Ana, J. Mater. Chem., 2007, 17, 1791 RSC.
- L. Shen, C. Yuan, H. Luo, X. Zhang, K. Xu and Y. Xia, J. Mater. Chem., 2010, 20, 6998 RSC.
- Y. Wang, H. Xu, X. Wang, X. Zhang, H. Jia, L. Zhang and J. Qiu, J. Phys. Chem. B, 2006, 110, 13835 CrossRef CAS PubMed.
- X. Song and L. Gao, J. Phys. Chem. C, 2008, 112, 15299 CAS.
- X. Zhao and L. Qi, Nanotechnology, 2012, 23, 235604 CrossRef PubMed.
- Y. Qin, F. Zhang, Y. Chen, Y. Zhou, J. Li, A. Zhu, Y. Luo, Y. Tian and J. Yang, J. Phys. Chem. C, 2012, 116, 11994 CAS.
- M. Agrawal, S. Gupta, A. Pich, N. E. Zafeiropoulos and M. Stamm, Chem. Mater., 2009, 21, 5343 CrossRef CAS.
- S. Xuan, W. Jiang, X. Gong, Y. Hu and Z. Chen, J. Phys. Chem. C, 2009, 113, 553 CAS.
- W. W. Wang, Y. J. Zhu and L. X. Yang, Adv. Funct. Mater., 2007, 17, 59 CrossRef CAS.
- M. K. Yu, J. Park and S. Jon, Theranostics, 2012, 2, 3 CrossRef CAS PubMed.
- Y. W. Zhang, M. Tang, X. Jin, C. S. Liao and C. H. Yan, Solid State Sci., 2003, 5, 435 CrossRef CAS.
- S. Chibowski and M. Paszkiewicz, Adsorpt. Sci. Technol., 2001, 19, 397 CrossRef CAS.
- S. Morariu and M. Bercea, Rev. Roum. Chim., 2011, 56, 545 CAS.
- M. Duval, Macromolecules, 2000, 33, 7862 CrossRef CAS.
- V. K. LaMer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 4847 CrossRef CAS.
- X. B. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed.
- C. Neinhuis and W. Barthlott, Ann. Bot., 1997, 79, 667 CrossRef.
- L. Jiang, Y. Zhao and J. Zhai, Angew. Chem., 2004, 116, 4438 CrossRef.
- Y. C. Park, Y. J. Chang, B. G. Kum, E. H. Kong, J. Y. Son, Y. S. Kwon, T. Park and H. M. Jang, J. Mater. Chem., 2011, 21, 9582 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The appearances and weights of the HMOS of TiO2, Fe2O3 and ZrO2, SEM images of hierarchical spheres of TiO2–Fe2O3, TiO2–Fe2O3–ZrO2 composites and their corresponding TEM/EDX maps, SEM image of TiO2 film by wet grinding fabrication process, SEM image of TiO2 powders after grinding, The infrared transmittance spectra, SEM images of TiO2 film using PEG of different molecular weights, SEM images of TiO2 film with different PEG mass ratio, the LaMer model explanation. See DOI: 10.1039/c4ra03164d |
| This journal is © The Royal Society of Chemistry 2014 |