Structures and morphologies of biocompatible and biodegradable block copolymers
Shaoyong Huangb and
Shichun Jiang*a
aSchool of Materials Science and Engineering, Tianjin University, Tianjin 300072, P. R. China. E-mail: scjiang@tju.edu.cn
bKey Laboratory of Polymer Eco-materials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
First published on 23rd May 2014
Abstract
Biocompatible and biodegradable block copolymers (BBCPs) have become increasingly important in polymer science, and have many potential applications in polymer materials. The structures of BBCPs, which are determined by the competition between crystallization, microphase separation, kinetics and processing, have a tremendous influence on the final properties and applications. In this review, the most recent advances are highlighted in the crystalline structures and morphologies of BBCPs with at least one crystalline block. Particular emphasis is placed on the influences of chemical composition, molecular architecture, crystallization pathway, and film thickness on the structures and morphologies of the block copolymers. The formation and the characteristics of the structures grown in the block copolymers are helpful for understanding the interplay between crystallization and phase segregation, morphologies, structural evolution and their applications.
1. Introduction
Block copolymers with biodegradability and biocompatibility have attracted more and more attention because of their potential applications in environmentally friendly and medical fields.1–11 Block copolymers are an interesting option, in which combinations of different chemical structures can be employed without macroscopic phase segregation.3,12,13 Most of the biocompatible and biodegradable polymers are semi-crystalline, such as poly(L-lactic acid) (PLLA), poly(D-lactic acid) (PDLA), poly(ethylene oxide) or poly(ethylene glycol) (PEO or PEG), poly(ε-caprolactone) (PCL), poly(p-dioxanone) (PPDX), poly(propene glycol) (PPG), poly(hydroxyl butyrate) (PHB), poly(butylene succinate) (PBS), poly(3-hydroxyalkanoate) (PHA), and poly(glycolic acid) (PGA). Usually, three kinds of block copolymers including AB diblock, ABA triblock and multi-arm block copolymers have been synthesized and investigated. Structures and morphologies of these block polymers resulting from crystallization and microphase separation determine the final properties, such as biodegradability, biocompatibility, optical and mechanical properties, by dominating microstructure, micro-domain morphology and overall crystallinity.14–18The blocks in block copolymers are usually immiscible and would undergo microphase separation under certain conditions,19,20 which drives molecular chains to self-assemble into a variety of ordered nanostructures, including lamellae, spheres, cylinders and gyroids, depending on its chemical composition and thermal pathway.21–29 The formation of these nanostructures is useful for the fabrication of functional materials, which can be applied in lithography, nanomaterials and opto-electronic devices.30–34 The Crystal thickness of a semi-crystalline block copolymer usually is in the range of nanoscale. The size is comparable with the dimension of the micro-domains induced by microphase separation of block copolymers. Therefore, the block copolymers exhibit considerable morphology richness, arising from the two main forces that can drive structure development: microphase separation between the blocks, which favors the formation of nano-domains, and the crystallization of the blocks, which favors the formation of alternating amorphous and crystalline layers.1
Most of the biocompatible and biodegradable polymers are crystallizable. Consequently, the biocompatible and biodegradable block copolymers are usually consisting of at least one crystalline block. Therefore, the resulting structures formed by the block copolymers depend on the location of the order–disorder transition temperature (TODT), the glass transition temperature of the non-crystalline block (Tg) and the crystallization temperature (Tc) of the crystalline block. When the melting temperature of one block is much higher than the other block, such as PLLA–PCL and PLLA–PEG block copolymers, either crystallization can overwrite any previous micro-domains formed by microphase separation for weakly segregated systems or homogeneous systems, or crystallization can be confined within the micro-domains for strongly segregated systems.35–39 For example, for crystallization of PLLA block in weakly segregated PLLA–PCL block copolymer, TODT > Tc > Tg, the initial crystallization occurs within the PLLA-rich micro-domains, and then the further crystallization breaks out the micro-domains, and a new structure is obtained driven by the crystallization of PLLA. In the same block copolymer, for the crystallization of PCL block, TODT > Tg > Tc, the crystallization of PCL block is confined in the micro-domains formed by microphase separation of the copolymer, crystallization and vitrification of PLLA block. When the melting temperatures of the two blocks in the copolymer are near enough, such as some PCL–PEO block copolymers, the situation can be even complicated, since the crystallization of one block and the structure formed can affect those of the other block. Moreover, a coincident crystallization phenomenon of both blocks may be obtained in some PEG–PCL block copolymers.40–42
In the present paper, the influences of chemical composition, molecular architecture, crystallization pathway, and film thickness on the structures of biocompatible and biodegradable block copolymers with at least one crystallizable block were reviewed.
2. Structures of biodegradable and biocompatible block copolymers (BBCPs)
2.1 Crystallization of miscible BBCPs
The structure of block copolymers is strongly depending on the chemical composition and crystallization kinetics. Based on the miscibility of the unlike blocks, the block copolymers can be generally clarified into weakly segregated and strongly segregated ones. There are two typical crystallization modes of polymer melting crystallization: break-out crystallization and confined crystallization. When the blocks of the block copolymers are weakly segregated, break-out crystallization will take place and lamellar structures with alternating crystalline–amorphous layers are usually formed.17,18,43–46 The lamellar crystalline structures have been observed in the structures of PLLA–PEO or PLLA–PEG,15–18,47–54 PEO–PCL or PEG–PCL,55–61 and PLLA–PCL diblock and triblock copolymers.62–64 When the blocks are strongly segregated, the crystallization of the minor component may be confined in micro-domains driven by microphase separation.Moreover, the crystalline structure of block copolymers is related to the fraction of each block and the relationship between three characteristic temperatures, TODT of the order–disorder transition temperature, Tg of the non-crystalline block, and Tc of the crystalline block. Generally, if the weight fraction of the minor component is lower than 20%, it will be difficult to crystallize.17,51,55–58,61,65–67 The crystallization of the crystallizable block will overwrite or be confined in the micro-domains, depending on the Tg of the non-crystalline component and the Tc of the crystallizable component. Break-out crystallization take place with Tc > Tg, and crystallization is confined for block copolymers with Tg > Tc within 1D-, 2D- and 3D-corresponding to lamellar, cylindrical, and spherical micro-domains.68
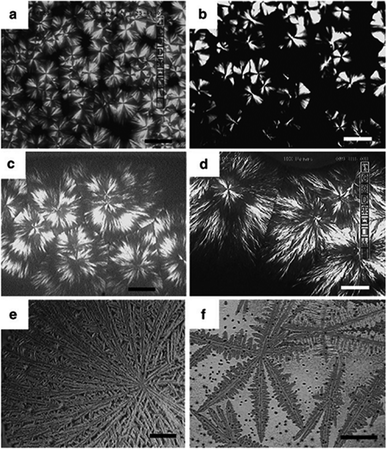
The melting point (Tm) and the crystallization temperature (Tc) of PLLA are ca. 160–180 °C and ca. 100–120 °C, respectively. The Tc of PLLA is higher than the Tg of PEO, PCL, and part of other biocompatible and biodegradable polymers. In BBCPs containing PLLA major segment, the PLLA crystallization drives microphase separation and would break-out the previously formed micro-domains.1,15–18 Spherulitic morphologies have been reported in BBPCs, e.g. PLLA–PEO, PLLA–PCL, PEO–PCL, PPDX–PCL block copolymers. Morphologies changed with composition and crystallization pathway, due to the interaction of microphase separation and crystallization process. Fig. 1 shows the morphologies of PLLA–PEO block copolymer driven by microphase separation and crystallization of PLLA. Spherulites were observed in low Tc range (at 90 °C and 100 °C in Fig. 1(a) and (b)). Ring-banded spherulites were formed at higher Tcs (at 110 °C and 115 °C in Fig. 1(c) and (d)). An transitional morphology of which the center is spherulite and the outer is dendrite was observed at 115 °C (Fig. 1(d)). At Tcs above 120 °C, dendritic super-structures were formed as shown in Fig. 1(e) and (f). The angle between the second branch and the main branch was less than 90° in Fig. 1(e), while at 125 °C in Fig. 1(f), the angle between the second (third) branch and the main (second) branch was almost 90°, indicating the influence of micro-domains of microphase separation driven by crystallization and PEO–PLLA segregation.
The morphological evolution with crystallization pathway (Tc) can be schematically illustrated in Fig. 2, which is associated with the microphase separation induced micro-domains and the break-out crystallization of PLLA block. With disorder to order transition, PEO-rich micro-domains and PLLA-rich micro-domains could be formed.1,17,18,52,69,70 Crystallization of PLLA starts in the PLLA-rich domains, because there are sufficient PLLA chains for initial growth of PLLA crystals .With consumption of the amorphous PLLA segments inside, the neighbor PLLA chains begin to diffuse onto the growth fronts, and break-out crystallization occurs. Because of the miscibility or weak segregation between PLLA and PEO blocks, amorphous PEO is rejected from PLLA crystalline lamellae, and distributed between PLLA lamellae and/or confined amorphous PLLA nano-domains.69–71 At low Tcs, crystallization of PLLA determines the final morphologies, and spherulitic structures are produced. At high Tcs, phase separation induces single crystal formation,17,52,72 and dendritic morphologies are obtained. The morphological evolution with Tc is also seen in block copolymers of PLLA–PCL, PCL–PEO (or PCL–PEG), PPDX–PCL.15–18,34–72
 | ||
| Fig. 2 Morphological evolution of melt-crystallized PLLA–PEO diblock copolymer as a function of crystallization temperature. | ||
The structural evolution may be related with different chain folding modes. Chain folding in block copolymers is a function of segregation strength of the unlike blocks,73 molecular weight of the crystalline block,74 and the crystallization temperature.75 Generally, polymer chains tend to fold parallel to the copolymer interphases as shown in Fig. 3a, when the block copolymers are strongly segregated, or the molecular weight of the crystalline block is small, or at low crystallization temperature ranges. Chain folding becomes perpendicular to the copolymer interphase as shown in Fig. 3b, when the block copolymers are weakly segregated, or the molecular weight of the crystalline block is large, or at high crystallization temperature ranges. The nano-structural evolution of the biodegradable block copolymers is helpful for applications in nanotechnology.76–78
 | ||
| Fig. 3 Schematic representation of chain folding and domain spacing of PLLA block and amorphous PEO at low ((a) 110 °C) and high ((b) 130 °C) crystallization temperatures. | ||
In crystalline–amorphous and crystalline–crystalline BBCPs, one crystalline block crystallizes and break-out the microphase separation induced micro-domains. The mode of microphase separation induced micro-domains would influence the final structure of the block copolymers. Fig. 4 shows the final morphologies of crystalline–amorphous PLLA30k–PEO5k block copolymer (30k and 5k correspond the molecular weight of each block). It is difficult for PEO5k to crystallize in PLLA30k–PEO5k block copolymer, because the volume fraction of PEO chains and the size of PEO-rich domains are too small to crystallize. Hexagonal and two peculiar dendritic morphologies are observed in PLLA30k–PEO5k block copolymers at high Tcs (120 and 130 °C). In Fig. 4a, there are two different sectors arranged alternately, which can be classified in terms of the crystal growth direction and chain-folding.1,17 In Fig. 4b and c, S-shaped structures are obviously seen, which is associated with two different growth modes of flat-on and edge-on, and the micro-domains formed by microphase separation and crystallization.17,69,72,79
 | ||
| Fig. 4 Optical micrographs in PLLA30k-b-PEO5k crystallized at (a) 120, (b) 130, (c) 130 °C. (J. Poly. Sci. Part B: Poly. Phys., 2008, 46, 1400–1411.) | ||
2.2 Confined crystallization of BBCPs
Confined crystallization is usually observed in strong segregated or crystalline/glassy block copolymers,80–84 and some crystalline-crosslinking rubbery block copolymers.85 The interface between the two immiscible micro-domains or the glassy wall of the glassy block have strong confinement on the crystalline block.Fig. 5 shows the atomic force microscopy images of double crystalline LA505EG505 (PLLA–PEG) diblock copolymer. The confined crystallization of single crystals was investigated. The crystallization process becomes more complicated in double crystalline block copolymers. The crystallization of LA505 takes place firstly, and the structures determined by microphase separation and crystallization of LA505 are vitrified by the glass transition of LA505. The crystalline and the glassy amorphous LA505 domains have strong confinement on crystallization of EG505. Actually, both blocks of LA505 and EG505 affect the crystallization behavior. The foregoing crystallization behavior of the PLLA severely constrains the crystallization of the PEG as shown the structures in Fig. 5.
Hexagonal shaped PLLA single crystals, which are the same as PLLA homopolymer, grow firstly from PLLA-rich micro-domains, as shown in Fig. 5a. At the early stage of PLLA crystallization, microphase separation of PLLA–PEG blocks has little effect on the nanoscale crystalline structures of PLLA, but the further crystallization of PLLA is influenced by microphase separation. As a result, the LA505 lamellar crystal of the nether layer on the substrate is star-shaped, which is different from the single crystal of PLLA homopolymer, indicating its growth is affected by the nano-domains of EG505 block and microphase separation driven by simultaneous crystallization. The growth rate of the first layer is much higher than that of the second layer and the third layer crystal, due to sufficient amorphous PLLA blocks and the short diffusion length. After the crystallization of PLLA completes, the film is cooled to 35 °C for the crystallization of EG505 block. PLLA crystals acts as nuclei of PEG block (EG505), and PEG crystals emerge at the edge of PLLA crystals, as shown in Fig. 5d. The final morphology of the LA505EG505 block copolymer changes into dendritic crystals because of the crystallization of the PEG block, as shown in Fig. 5e and f. Crystallization of PLLA and microphase separation make PEG phase form nano- and micro-domains between PLLA crystal lamellae, the interfaces of polymer–air and polymer–substrate, and the bundles of PLLA crystals.1,17,18,27,29,51–54,69,70The crystallization of PEG block is confined by the glassy PLLA, and the crystal of PEG block grows epitaxially on the PLLA crystal. The (1 0 4) plane of the PEG crystal is parallel to the (0 0 1) plane of the PLLA crystal and the substrate.69
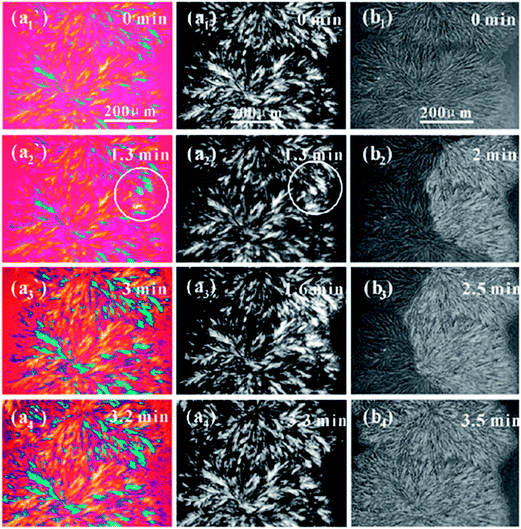
Fig. 6 shows the confined crystallization in symmetric PLLA–PEG block copolymer (LA5kEG5k) via two steps crystallization. At the beginning of 110 °C, microphase separation has been absence after disorder to order transition. When the sample isothermally crystallized at 110 °C, only the PLLA component crystallize, and a dendritic morphology is observed as shown in Fig. 6a1, a1′ or b1. The PEG component is still in molten state at 110 °C. On the one hand, the melted PEG block is in favor of PLLA crystallization, due to decreasing PLLA melt viscosity. On the other hand, the nanoscale and microscale domains of PEG suppress PLLA crystallization. In the crystallization process of PLLA block, the amorphous PEG block is eliminated from the growth front of PLLA crystals, resulting in nanoscale domains of PEG block. The crystallization of PLLA block induces a new segregated structure, which breaks up the previous structure formed by disorder to order transition of the block copolymer. When the PLLA–PEG block copolymer subsequently crystallize at 30 °C, PEG blocks started to crystallize, and a grain-like brighter area appear at the boundary of the foregoing PLLA spherulites (as shown in Fig. 6a2 and a2′, marked by the circle). The brighter area finally covers the whole PLLA spherulite, as shown in Fig. 6a2–a4, a2′–a4′ and b2–b4. The PEG crystals grow in the regions of interlamellar of PLLA crystals confirmed by SAXS data, and fill into the amorphous spaces inside the PLLA spherulites.70 Though PEG blocks crystallize in the block copolymer, morphological feature of the block copolymer do not change at all on the micrometer scale, as shown in Fig. 6a1–a4 and b1–b4. However, the crystallization of PEG blocks significantly enhances the contrast of the foregoing PLLA spherulites by changing the optical property of the block copolymer, and it affects the structure in nanometer scale.
The spherical, lamellar and cylindrical micro-domains formed by microphase separation and/or the forgoing crystallization confine the crystallization of the second crystalline component. It should be noted that, although the morphology of the crystallized block copolymer is the same before and after confined crystallization, the properties, such as optical, mechanical, rheological and biodegradable properties,69,86–88 are indeed altered due to the crystallization.
Both break-out and confined crystallization can be utilized to regulate the structure and properties of BBCPs, via controlling chemical composition, molecular weight, crystallization kinetics.
2.3 Eutectic crystal of double crystalline BBCPs
In weakly segregated block copolymers, eutectic structure can be obtained if the two different crystalline blocks have comparable Tcs and Tgs, because of the connectivity of the blocks in molecular level.14,28,40–42,55–59,65–67,84–89 In crystalline–crystalline PCL–PEG (or PCL–PEO) block copolymers, PEO and PCL lamellae occupy the same crystallites when comparable fractions of each component were present.14,40–42,84,89 Jiang et al. observed the ring-banded spherulite of the crystalline–crystalline PEO–PCL diblock copolymer with minor weight fraction of PEO block of 0.18. It is found that the PCL blocks crystallize firstly, and PEO blocks subsequently crystallize. PEO block crystallize in a soft-confinement, and concentric circles are not observed due to the minority fraction of PEO block.14 With weight fraction of PEO component of ∼0.40 and ∼0.5, a unique double concentric spherulite can be obtained in PCL–PEO block copolymers,14,40–42,84,90 as shown in Fig. 7. He et al. used temperature-dependent FTIR to analyze the crystallization process of PEG–PCL.40 It is found that the PCL block melted later in the heating process and crystallized firstly in the slow cooling process. Consequently, PCL spherulites are firstly observed, as shown in Fig. 7a, and the growth rate of PCL is ca. 2.8 μm s−1. Just after several seconds, a sudden birefringent change (marked by arrows in Fig. 7b) within one spherulite is observed, and the growth rate of the new birefringence within the spherulite was 15.1 μm s−1. The new birefringence triggered the growth of a new outer spherulite from the existing front of the inner spherulite (Fig. 7c and d), resulting in a concentric spherulite. It is confirmed by in situ micro-beam FT-IR that the new outer spherulite is PEG, and the growth rate of PEG is 4.4 μm s−1. The decrease of the growth rate from 15.1 μm s−1 to 4.4 μm s−1 indicates the soft confinement on crystallization of PEG blocks. | ||
| Fig. 7 Real-time POM micrographs of PEG50–PCL50. The specimen was melted at 80 °C for 5 min and then quenched to 38 °C at 40 °C min. (Biomacromolecules, 2006, 7, 252–258). | ||
Moreover, the crystallization behavior and structure of PEO-b-PCL are significantly composition dependent. Fig. 8 shows the time resolved SAXS and WAXS measurements of PEO–PCL block copolymers collected during the isothermal crystallization at 40 °C. In the copolymers with the weight fraction of PCL of 0.23–0.87, both blocks can crystallize. When the weight fraction of PCL is 0.36 or less, PEO crystallized first. On the contrary, PCL crystallized first when the weight fraction of PCL is 0.43 or more. When the length of the PEG and PCL block is comparable (0.43–0.5), concentric spherulite can be obtained, but the concentric spherulite is not eutectic crystal of PEO and PCL, but originates from step crystallization of PEG and PCL blocks. When the length of the two blocks is quite different, only spherulite can be observed. The long period of the block copolymer increases gradually as the weight fraction of PCL increasing from 0 to 0.50, due to the increase of the crystalline PCL layer thickness. With further increasing the weight fraction of PCL from 0.50 to 0.87, long period of the copolymer reduced significantly, owing to the depression of the crystalline PEO layer thickness.
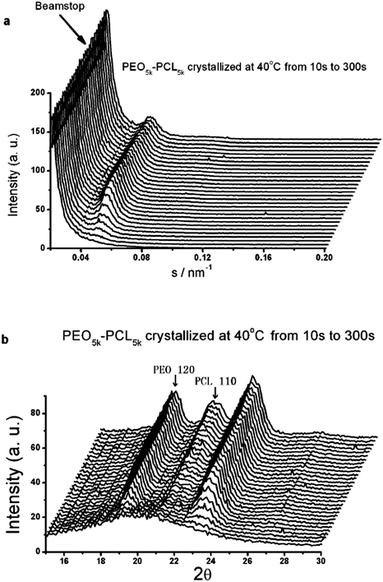 | ||
| Fig. 8 Simultaneous (a) SAXS and (b) WAXS profiles collected during isothermal crystallisation of PEO5k–PCL5k at 40 °C.90 (Eur. Phys. J. E: Soft Matter Biol. Phys., 2008, 27, 357–364). | ||
Eutectic crystal was observed in symmetric PEG5000–PCL5000 block copolymer-hexanol solution with block molecular weights of ∼5000.42 Fig. 9 shows the AFM micrographs of PEG5000, PCL8000, and PEG5000-PCL5000 single crystals grown from dilute hexanol solution. PEG5000 single crystal is square shaped as shown in Fig. 9a, and PCL8000 single crystal is hexagonal shaped as shown in Fig. 9(b).91–93 PEG5000–PCL5000 single crystal exhibits an elongated hexagonal shape as shown in Fig. 9c. The size of PEG5000–PCL5000 single crystals is much larger than those of PEG5000 and PCL5000 single crystals grown under the same conditions. The crystalline structure shown in Fig. 9c exhibits coexistence of the two single-crystal layers formed from the two blocks. The PCL blocks crystallize first to form a screw-dislocated lamella with a shape of truncated lozenge, and then the PEG chains fold themselves on the two surfaces of the PCL lamella to achieve a three-layer structure. It is not easy to form eutectic crystals in block copolymers, due to the immiscibility of the unlike blocks. Up to now, eutectic crystals are only observed in symmetric PEG–PCL block copolymers with low molecular weight, and in stereocomplex of polymers blends.94,95
 | ||
| Fig. 9 AFM height (left) images and phase (right) images of (a) PEG5000, (b) PCL8000, and (c) PEG5000 PCL5000. (Macromolecules, 2006, 39, 3717–3719). | ||
Solution grown single crystals of a series of double crystalline diblock copolymers PLLA–PCL were reported by Casas et al.96 Crystalline morphologies depend on the crystallization temperature and the composition. PLLA blocks crystallize firstly, and rendered lozenge, truncated and spindle-shaped crystals can be formed, as shown in Fig. 10. The edges of the crystal morphologies are thicker than that in the center, due to PLLA overgrowths in their periphery. An overgrowth of irregular PCL crystals can be also observed during its crystallization. Complex morphologies constituted by lamellar crystals of PCL and PLLA blocks were developed at intermediate temperatures (70–65 °C, Fig. 10). Electron diffraction patterns (Fig. 10) showed the growth of PCL and PLLA crystals.
2.4 Stereocomplexation of enantiomeric block copolymer
Stereocomplexation has been known to take place upon the mixing of two polymers that have the same composition but with different stereo-configurations, e.g. between poly(L/D-2-hydroxybutanoic acid),94,95 isotactic/syndiotactic poly(methyl methacrylate) (PMMA)97 and poly(γ-benzyl glutamate),98–100 PLLA–PDLA.101–106 The structure of PLLA α-crystal is pseudo-orthorhombic, as shown in Fig. 11(a),107–111 and the corresponding crystal structure of PDLA is enantiomeric to PLLA crystal. The structure of PLA stereocomplex is shown in Fig. 11(b) and (c).112 It shows that the lattice containing a PLLA and a PDLA chain with a 31 helical conformation, and they together form an equilateral-triangle-shaped single crystal of PLA stereocomplex. The melting temperature of PLA stereocomplex is 50–60 °C higher than that of PLA homopolymer crystal, and the heat-distortion temperature of PLA stereocomplex is above 100 °C. Fig. 12 shows the thermal properties of PLA stereocomplex of PLLA–PDLA block copolymer (sb-PLA) and of PLLA–PDLA blend (melt-blend). It can be seen that the melting point of sb-PLA is higher than that of melt-blend, indicating PLLA–PDLA block copolymer enhances the crystallinity and crystal structure of PLA stereocomplex, because the enantiomeric PLLA and PDLA chains are chemically linked and mixed in molecular level. Furthermore, the structure of PLA stereocomplex is related with molecular weight, PLLA–PDLA ratio, and chain architecture.112 Fig. 12(b) shows the WAXD patterns of PLA homopolymer crystal and PLA stereocomplex crystal. The WAXD pattern of PLA stereocomplex is different from that of PLA homopolymer crystal. In melt-blend, the peak of PLA homopolymer crystal can be observed, due to microscopic phase separation and inhomogeneous distribution of PLLA and PDLA. Generally, only sb-PLA stereocomplex is obtained in symmetric PLLA–PDLA diblock copolymer with the molecular weight of <60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.113 PLLA–PDLA stereocomplexation may undergo microscopic phase separation in a PDLA-rich and a PLLA-rich phase, which will induce homopolymer crystallization, especially when the molecular weight is high (e.g. 100
000 g mol−1.113 PLLA–PDLA stereocomplexation may undergo microscopic phase separation in a PDLA-rich and a PLLA-rich phase, which will induce homopolymer crystallization, especially when the molecular weight is high (e.g. 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).113–115
000 g mol−1).113–115
Stereo multiblock poly(lactic acid)s (PLA)s and stereo diblock poly(lactic acid) (DB) with a wide variety of block length of 15.4–61.9 lactyl units are synthesized, and the effects of block length sequence on crystallization and spherulite growth are investigated.116 Only stereocomplex crystallites are formed in the stereo multiblock PLAs and DB, irrespective of the block length and crystallization temperature. The melting points the stereo multiblock PLAs and DB increase with increasing block length, but are less than those of PLLA–PDLA blends, as shown in Fig. 13. The spherulite growth rates and overall crystallization rates of the stereo multiblock PLAs and DB increase with increasing block length and are lower than that of PLLA–PDLA blend.
Diblock and multiblock PLLA–PDLA copolymers have been synthesized, and the PLLA–PDLA composition can be widely changed. The crystallinity and properties can be controlled by changing the number of blocks, the block length, and the enantiomeric ratio in a chain. In melt crystallization of PLLA–PDLA block copolymer, spherulitic morphology can be observed.113,114 Up to now, there are only a few researches on the structure and morphology of PLLA–PDLA block copolymers,106,106,115 and should be extensively investigated, because they are close related with the properties and applications.
2.5 Structures of multiblock copolymers
The crystallization and structure of multiblock copolymers become complicated, because of their complicated structure of polymer chains and the confinement from the chemical bonds of the unlike blocks. The architectures of multiblock copolymers can be classified into linear, star-shaped and branched chains. The main application of multiblock copolymers is used as biomaterials, such as shape memory polymers.116–122 Linear multiblock copolymers comprising of crystallizable poly(butylene succinate) (PBS) hard segments and poly(ethylene glycol) (PEG) (PBS–PEG) soft segments, and poly(ethylene succinate)-b-poly(butylene succinate) (PES-b-PBS) were synthesized, and the crystallization and structures were investigated.117–119 The double-crystalline multiblock copolymers formed separated crystalline domains, which determined the permanent shape and temporary shape respectively. The Tm of PEG segment varied with its content ranging from 27.54 to 51.04 °C, acting as the transition temperature. PBS and PEG segments, PES and PBS segments of the multiblock copolymers are miscible over the entire composition range in the amorphous state. However, PBS, PES and PEG segments in the copolymers can form their own crystals. The crystallization and structure of each segment show strong dependence on its content. Moreover, it is showed that PEG crystalline domains disturb or restrict the crystal growth of PBS lamellae.117 PBS segment crystallizes firstly, and the PBS crystalline domains confine or affect the crystallization of PES segment. The crystallization of PES segment may break-out and/or is confined within the PBS crystalline domains, and they both determine the final crystalline structure, as shown in Fig. 14.118The macromolecular architecture of star-shaped polymers is the unique three-dimensional shaped, and they always exhibit useful properties in comparison with linear polymers, and are expected to display peculiar structures and morphologies. Star-shaped multiblock copolymers of PCL–PLLA120 and PCL–PEO121 with different arm number were synthesized and investigated. Star-shaped architecture does not have obvious effect on the crystalline morphologies, though star-shaped polymers have a lower glass-transition temperature, melting temperature, and crystallinity than those of a linear analogue.
As shown in the Fig. 15(A), spherulitic morphologies are observed, indicating 6-armed PCL homopolymer (6sPCL) possesses good crystallizability.120 The AFM image of 6 armed PCL–PLLA (6sPCL-b-PLLA) multiblock copolymer exhibits tiny morphology (Fig. 15(B)), which should be attributed to the outer PLLA block restricting the crystallization of PCL segment. In all, both the star-shaped architecture and the composition control the crystal growth and morphology.
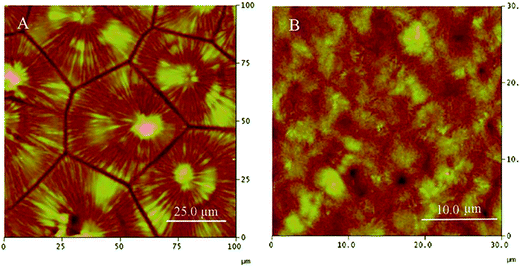 | ||
| Fig. 15 AFM images of crystallized 6-armed PCL and 6-armed PCL–PLLA block copolymers. (J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5063–5071.). | ||
Crystallization and self-assembly of branched multiblock BBCPs have attracted increasing attention due to the biomedical applications.122 H-shaped block copolymers of poly(L-lactide)-b-(poly(2-(N,N-dimethylamino) ethyl methacrylate)-b-)poly(ε-caprolactone)(-b-poly(2-(N,N-dimethylamino)ethylmethacrylate))-b-poly(L-lactide) (PLLA-b-(PDMAEMA-b-)PCL(-b-PDMAEMA)-b-PLLA) were synthesized,123 The crystallinity of PCL and PLLA decreased due to the presence of amorphous PDMAEMA segments in the H-shaped copolymers, as shown in Fig. 16. Maltese cross patterns are not found, and the crystalline structure presents flower like morphology for PLLA-B-(N3-)PCL(-N3)-B-PLLA (Fig. 16c). This can be attributed to the decrease of crystallizability of PCL and PLLA segments. PLLA-b-(PDMAEMA-b-)PCL(-b-PDMAEMA)-b-PLLA presented a more complicated spherulitic morphology. The formation of the banded spherulites (as shown in Fig. 16d) is related to lamellar twisting along the radius orientation of the PCL spherulite, which can be attributed to the presence of amorphous PDMAEMA and PLLA crystals. Therefore, the H-shaped copolymer film has better hydrophilicity than PCL or triblock copolymer of PLLA-b-(N3-)PCL(-N3)-b-PLLA. The unique H-shaped amphiphilic terpolymers, which are composed of biodegradable and biocompatible PCL and PLLA components and intelligent and biocompatible PDMAEMA component, have potential applications in biomedical fields.
 | ||
| Fig. 16 POM of (a) PCL, (b) N3-PCL-N3, (c) PLLA-b-(N3A)PCL-(N3-)-b-PLLA, (d) PLLA-b-(PDMAEMA-b-)-PCL(-b-PDMAEMA)-b-PLLA. (J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2541–2552). | ||
2.6 Structures of BBCP thin films
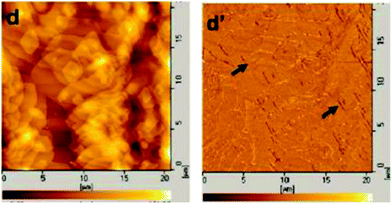
Different from bulk materials, microphase separation can also occur in BCP thin films. Except crystallization and microphase separation, the interactions of BCP–substrate interface and air–BCP interface also play an important role in the crystallization behavior and structure formation of BCP thin film. By changing the crystallization pathway, composition, film thickness, and even substrate, the structure of BCP thin film can be regulated. Film thickness is an important factor affecting the structure of block copolymer. In thin films, crystallization and microphase separation are confined. Wetting/dewetting process in thin films may take place, and will affect the structure of the block copolymer by affecting crystallization and microphase separation.124–126Fig. 17 shows the crystalline morphologies of PLLA–PEO block copolymer thin films. Hexagonal dendritic morphology which is composed of two sectors arranged alternately, was observed in thin films of 400 nm–200 nm, as shown in Fig. 17a and b.1 Similar morphology was also observed in PLLA30k–PEO5k block copolymer.17 In the adjacent two sectors, the chain folding and growth direction were different. In thin film of ∼100 nm, lozenge-shaped lamellae were formed. The stack and the grow crystals are oriented as shown in Fig. 17c. Furthermore, the holes in the center of the lozenge-shaped morphology can be observed, indicating possible de-wetting occur during annealing process. Holes or islands are formed after de-wetting of the thin films, leading to an increase in local polymer chains concentration and orientation of polymer chains at the edge of the holes or islands. Consequently, it becomes easy for the polymer chains to crystallize at the edge of the holes and islands, and then initial crystallization and morphology are observed at the edge of the holes or islands.1,127–129
Fig. 18 shows the detailed crystalline morphologies of the PLLA16k-b-PEO5k copolymer thin films. Lozenge-spiral dislocation (marked by arrows), lozenge-multilayer was formed in the growth direction. The spacing of lamellae is identical with that of bulk PLLA–PEO, but the size of the lozenge-shaped crystal in thin film is smaller than that of bulk PLLA–PEO. The effect of substrate and 2D confinement of thin film play important role on the peculiar morphology. The morphology of block copolymers in thin film is strongly dependent on the film thickness. Crystallization is severely suppressed due to 2D confinement of thin film, resulting in peculiar structure and morphology.130,131 Using a so called micro-evaporation technique, mesoscale dendritic structures were observed in thin films.132–134 In ultrathin films less than 50 nm, Zhao et al. observed dendritic and fiber-like morphologies of PCL–PEO block copolymer as shown in Fig. 19, which is associated with low molecular weight of PCL–PEO block copolymer, and the interaction between microphase separation and crystallization.134
 | ||
| Fig. 18 AFM height (left) and phase (right) images of PLLA16k-b-PEO5k copolymer thin films. (Langmuir, 2009, 25(22), 13125–13132.). | ||
 | ||
| Fig. 19 AFM height images of PEO(4k)-b-PCL(20k) films crystallized at room temperature at film thicknesses of (a) 14, (b) 10 nm. The bar corresponding 2 μm. (Polymer, 2011, 52, 2085–2093). | ||
2.7 Micellar morphology of BBCPs
The self-assembly of block copolymers which contain at least one crystalline block has been another important area of micelle research.135–146 The understanding of the assembly morphology of amphiphilic block copolymers with a crystalline core structure is still considerably poor, and the improved insight is highly desirable. Nano-sized polymer crystals can acts as seeds for single crystals with regular shape, or for micelles with large size. Block copolymer micelles are generally spherical nano-metric particles or cylindrical nanotube that possess core–shell architecture. They originate from the self-assembly of amphiphilic block copolymers in a selective solvent above a threshold concentration, referred to as the critical aggregation concentration.137Monodisperse stereocomplex block copolymer micelles were obtained through the self-assembly of equimolar mixtures of PEG–PLLA–PEG and PEG–PDLA–PEG in water, as shown in Fig. 20.137 These micelles possessed partially crystallized cores of PLA stereocomplex, and the mean diameter can be regulated by changing PLLA and PDLA content. They exhibited kinetic stability and re-dispersion properties superior to micelles prepared with isotactic or racemic polymers alone. It indicates that stereocomplex has advantages for the formation of stabilized water-soluble nanoparticles.
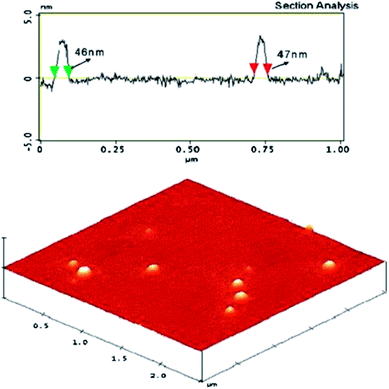 | ||
| Fig. 20 AFM image of stereocomplex PM composed of an equimolar mixture of PEG-b-PDLA72 and PEG-b-PLLA73. Imaging (Nano Lett., 2005, 5 (2), 315–319). | ||
A hollow structure such as nanotubes and vesicles play an important role in biological systems where they provide closed chambers for reaction, scaffolding and packing of proteins. Wu et al. synthesized symmetric or asymmetric PEG–PLA–PEG triblock copolymers. Various architectures such as nanotubes, polymersomes and spherical micelles by self-assemble in aqueous solution were observed, as shown in Fig. 21.138–140 The changes of morphology and size depended on the chain structure of copolymers.
The self-assembling aggregates of the biodegradable amphiphilic triblock copolymers can be schematically illustrated as Fig. 22, (a) spherical polymersomes and (b) cylindrical nanotubes.138–146 BBCPs consisting of methoxy PEG (mPEG) as a hydrophilic block and either crystalline P(CL-LLA) or amorphous P(CL-DLLA) as a hydrophobic block.141 Investigations were focused on the effect of the crystalline nature of the core-forming block on the formation of the micelles. Amorphous P(CL-DLLA)-b-mPEG block copolymers adopt a spherical shaped micelles. In contrast, crystalline P(CL-LLA)-b-mPEG block copolymers exhibit a cylindrical shaped micelles. The crystallization of the core-forming blocks of P(CL-LLA) is the driving force for the cylindrical morphology.
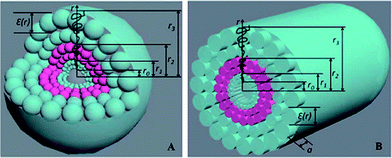 | ||
| Fig. 22 Schematic presentation of self-assembling aggregates from triblock copolymers: (a) polymersomes and (b) nanotubes. (Nanoscale, 2013, 5, 9010–9017). | ||
3. Conclusion and outlook
Biocompatible and biodegradable block copolymers can be miscible, weakly or strongly segregated depending on the compatibility and segregation between the unlike blocks. Competition of crystallization and microphase separation, and/or other driving force can lead to more abundant structures for BBCPs with at least one crystalline block. Some unique structures and morphologies resulting from break-out and confined crystallization, controlled growth of thin film crystallization, eutectic crystal of double crystalline blocks, stereocomplexation of enantiomeric isomers, and micelles with a crystalline core, can be observed for crystalline–amorphous, crystalline–crystalline diblock, triblock and multiblock copolymers. The assembled structures of the semicrystalline BBCPs can be regulated by changing chemical composition, chain architecture, crystallization pathway, film thickness, and the other internal and external factors.Structures and morphologies of semicrystalline BBCPs in bulk material have been extensively studied and well understood in diblock, triblock and multiblock copolymers. Researches on the relationship among the chemical composition, macromolecular architectures, thermal history, physical properties, mechanical performance, biodegradability and biocompatibility of BBCPs are frequently neglected. Meanwhile, the controlled nano-scale structures, the orientation of lamellar crystals, the relationship between structure and function of BBCPs, crystallization kinetics in ultra-thin films, confined crystallization from crystal lamellae and glass state should be more and further studied. Extensive studies are need for nano-structures and microscopic morphology of stereocomplex, including PLLA–PDLA, PLLA–PD2HB, PDLA–PL2HB diblock and multi-block copolymers. Controlled growth and micro-structure, growth kinetics of semicrystalline micelles of BBCPs are of importance for applications in biomedical area, and should be extensively studied.
4. Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21374077, 51173130 and 51103151).References
- S. Y. Huang, S. C. Jiang, X. S. Chen and L. J. An, Dendritic Superstructures and Structure Transitions of Asymmetric Poly(L-lactide-b-ethylene oxide) Diblock Copolymer Thin Films, Langmuir, 2009, 25(22), 13125–13132 CrossRef CAS PubMed.
- L. S. Nair and C. T. Laurencin, Biodegradable polymers as biomaterials, Prog. Polym. Sci., 2007, 32, 762–798 CrossRef CAS PubMed.
- R. V. Castillo and A. J. Müller, Crystallization and morphology of biodegradable or biostable single and double crystalline block copolymers, Prog. Polym. Sci., 2009, 34, 516–560 CrossRef CAS PubMed.
- N. Kumar, M. Ravikumar and A. Domb, Biodegradable block copolymers, Adv. Drug Delivery Rev., 2001, 53, 23–44 CrossRef CAS.
- T. Kissel, Y. Li and F. Unger, ABA-triblock copolymers from biodegradable polyester A blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery system for proteins, Adv. Drug Delivery Rev., 2002, 54, 99–134 CrossRef CAS.
- A. Harada and K. Kataoka, Supra-molecular assemblies of block copolymers in aqueous media as nanocontainers relevant to biological applications, Prog. Polym. Sci., 2006, 31, 949–982 CrossRef CAS PubMed.
- J. H. Kim, K. Park, H. Y. Nam, S. Lee, K. Kim and I. C. Kwon, Polymers for bioimaging, Prog. Polym. Sci., 2007, 32, 1031–1053 CrossRef CAS PubMed.
- M. Martina and D. W. Hutmacher, Biodegradable polymers applied in tissue engineering research: a review, Polym. Int., 2007, 56, 145–157 CrossRef CAS.
- Z. Rzaev, S. Dinçer and E. Piskin, Functional copolymers of N-isopropylacrylamide for bioengineering applications, Prog. Polym. Sci., 2007, 32, 534–595 CrossRef CAS PubMed.
- N. Rapoport, Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery, Prog. Polym. Sci., 2007, 32, 962–990 CrossRef CAS PubMed.
- D. Discher, V. Ortiz, G. Srinvas, M. Klein, Y. Kim and D. Christian, Emerging applications of polymersomes in delivery: from molecular dynamics to shrinkage of tumors, Prog. Polym. Sci., 2007, 32, 838–857 CrossRef CAS PubMed.
- I. W. Hamley, Crystallization in block copolymer, Adv. Polym. Sci., 1999, 148, 113–137 CrossRef CAS.
- A. J. Müller, V. Balsamo and M. L. Arnal, Nucleation and crystallization in diblock and triblock copolymers, Adv. Polym. Sci., 2005, 190, 1–63 CrossRef.
- S. C. Jiang, C. He, L. J. An, X. Chen and B. Z. Jiang, Crystallization and ring-banded spherulite morphology of poly(ethylene oxide)-block-poly(ε-caprolactone) diblock copolymer, Macromol. Chem. Phys., 2004, 205, 2229–2234 CrossRef CAS.
- I. Rashkov, N. Manolova, S. M. Li, J. L. Espartero and M. Vert, Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with short poly(L-lactic acid) chains, Macromolecules, 1996, 29, 50–56 CrossRef CAS.
- C. G. Mothé, W. S. Drumond and S. H. Wang, Phase behavior of biodegradable amphiphilic poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide), Thermochim. Acta, 2006, 445, 61–66 CrossRef PubMed.
- S. Y. Huang, S. C. Jiang, X. Chen and L. J. An, Crystallization and Morphology of Poly(ethylene oxide-b-lactide) Crystalline-Crystalline Diblock Copolymers, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 1400–1411 CrossRef CAS.
- J. Sun, Z. Hong, L. Yang, Z. Tang, X. Chen and X. Jing, Study on crystalline morphology of poly(L-lactide)-poly(ethylene glycol) diblock copolymer, Polymer, 2004, 45, 5969–5977 CrossRef CAS PubMed.
- I. W. Hamley, The physics of block copolymers, Oxford University Press, Oxford, 1998 Search PubMed.
- N. Hadjichistidis, S. Pispas and G. Floudas, Block copolymers: synthetic strategies, physical properties and applications, Wiley, New Jersey, 2003 Search PubMed.
- W. N. He and J. T. Xu, Crystallization assisted self-assembly of semicrystalline block copolymers, Prog. Polym. Sci., 2012, 37, 1350–1400 CrossRef CAS PubMed.
- F. S. Bates and G. H. Fredrickson, Block copolymers: designer soft materials, Phys. Today, 1999, 52, 32–38 CrossRef CAS PubMed.
- J. K. Kim, S. Y. Yang, Y. Lee and Y. Kim, Functional nanomaterials based on block copolymer self-assembly, Prog. Polym. Sci., 2011, 35, 1325–1349 CrossRef PubMed.
- Y. L. Loo, R. A. Register and A. J. Ryan, Polymer crystallization in 25 nm spheres, Phys. Rev. Lett., 2000, 84, 4120–4123 CrossRef CAS.
- V. R. Tirumala, A. Romang, S. Agarwal, E. K. Lin and J. J. Watkins, Well Ordered Polymer Melts from Blends of Disordered Triblock Copolymer Surfactants and Functional Homopolymers, Adv. Mater., 2008, 9999, 1–6 Search PubMed.
- L. Zhu, S. Z. D. Cheng, P. Huang, Q. Ge, R. P. Quirk, E. L. Thomas, B. Lotz, B. S. Hsiao, F. Yeh and L. Liu, Nanoconfined Polymer crystallization in the hexagonally perforated layers of a self-assembled PS-b-PEO diblock copolymer, Adv. Mater., 2002, 14, 31–34 CrossRef CAS.
- J. Lee, Y. H. Bae, Y. S. Sohn and B. Jeong, Thermogelling aqueous solutions of alternating multiblock copolymers of poly(L-lactic acid) and poly(ethylene glycol), Biomacromolecules, 2006, 7, 1729–1734 CrossRef CAS PubMed.
- J. Zhang, L. Q. Wang, H. Wang and K. Tu, Micellization phenomena of amphiphilic block copolymers based on methoxy poly(ethylene glycol) and either crystalline or amorphous poly(caprolactone-b-lactide), Biomacromolecules, 2006, 7, 2492–2500 CrossRef CAS PubMed.
- J. Fu, B. Luan, X. Yu, Y. Cong, J. Li, C. Y. Pan, Y. C. Han, Y. M. Yang and B. Y. Li, Self-assembly of crystalline coil diblock copolymer in solvents with varying selectivity: from spinodal-like aggregates to spheres, cylinders, and lamellae, Macromolecules, 2004, 37, 976–986 CrossRef CAS.
- A. Harada and K. Kataoka, Supramolecular assemblies of block copolymers in aqueous media as nanocontainers relevant to biological applications, Prog. Polym. Sci., 2006, 31, 949–982 CrossRef CAS PubMed.
- Q. Li, J. He, E. Glogowski, X. Li, J. Wang, T. Emrick and T. O. Russell, Responsive Assemblies: Gold Nanoparticles with Mixed Ligands in Microphase Separated Block Copolymers, Adv. Mater., 2008, 9999, 1–5 Search PubMed.
- J. Kim, K. Park, H. Y. Nam, S. Lee, K. Kim and I. C. Kwon, Polymers for bioimaging, Prog. Polym. Sci., 2007, 32, 1031–1053 CrossRef CAS PubMed.
- Z. M. O. Rzaev, S. Dinçer and E. Piskin, Functional copolymers of N-isopropylacrylamide for bioengineering applications, Prog. Polym. Sci., 2007, 32, 534–595 CrossRef CAS PubMed.
- U. Jeong, H. Kim, R. L. Rodriguez, I. Y. Tsai, C. M. Stafford, J. Kim, C. J. Hawker and T. P. Russell, Asymmetric Block Copolymers with Homopolymers: Routes to Multiple Length Scale Nanostructures, Adv. Mater., 2002, 14, 274–276 CrossRef CAS.
- P. Rangarajan, R. A. Register, D. H. Adamson, L. J. Fetters, W. Bras, S. Naylor and A. J. Ryan, Dynamics of structure formation in crystallizable block copolymers, Macromolecules, 1995, 28, 1422–1428 CrossRef CAS.
- A. J. Ryan, I. W. Hamley, W. Bras and F. S. Bates, Structure development semicrystalline diblock copolymers crystallizing from the ordered melt, Macromolecules, 1995, 28, 3860–3868 CrossRef CAS.
- S. Nojima, Y. Akutsu, A. Washino and S. Tanimoto, Morphology of melt-quenched poly(epsilon-caprolactone)-block-polyethylene copolymers, Polymer, 2004, 45, 7317–7324 CrossRef CAS PubMed.
- V. T. Lipik, J. F. Kong, S. Chattopadhyay, L. Widjaja, S. S. Liow, S. S. Venkatraman and M. J. M. Abadie, Thermoplastic biodegradable elastomers based on e-caprolactone and L-lactide block co-polymers: A new synthetic approach, Acta Biomater., 2010, 6, 4261–4270 CrossRef CAS PubMed.
- I. W. Hamley, V. Castelletto, R. V. Castillo, A. J. Müller and C. M. Martin, Crystallization in poly(L-lactide)-b-poly(epsilon-caprolactone) double crystalline diblock copolymers: a study using X-ray scattering, differential scanning calorimetry, and polarized optical microscopy, Macromolecules, 2005, 38, 463–472 CrossRef CAS.
- C. He, J. Sun, T. Zhao, Z. Hong and X. Chen, Formation of a unique crystal morphology for the poly(ethylene glycol)-poly(ε-caprolactone) diblock copolymer, Biomacromolecules, 2006, 7, 252–258 CrossRef CAS PubMed.
- C. He, J. Sun, J. Ma, X. Chen and X. Jing, Composition dependence of the crystallization behavior and morphology of the poly(ethylene glycol)-poly(ε-caprolactone) diblock copolymer, Biomacromolecules, 2006, 7, 3482–3489 CrossRef CAS PubMed.
- J. Sun, X. Chen, C. He and X. Jing, Morphology and structure of single crystals of poly(ethylene glycol)-poly(ε-caprolactone) diblock copolymers, Macromolecules, 2006, 39, 3717–3719 CrossRef CAS.
- S. Nojima, K. Kato, S. Yamamto and T. Ashida, Crystallization of block copolymers. 1. Small angle X-ray scattering study of an epsilon-caprolactone butadiene diblock copolymer, Macromolecules, 1992, 25, 2237–2242 CrossRef CAS.
- A. Ryan, I. Hamley, W. Bras and F. Bates, Structure development in semicrystalline diblock copolymers crystallizing from the ordered melt, Macromolecules, 1995, 28, 3860–3868 CrossRef CAS.
- P. Rangrajan, R. Register, L. Fetters, W. Bras, S. Naylor and A. Ryan, Crystallization of a weakly segregated polyolefin diblock copolymer, Macromolecules, 1995, 28, 4932–4938 CrossRef.
- G. Floudas, R. Ulrich and U. Wiesner, Microphase separation in poly(isoprene-b-ethylene oxide) diblock copolymer melts. 1. Phase state and kinetics of the order-to-order transition, J. Chem. Phys., 1999, 110, 652–663 CrossRef CAS PubMed.
- S. Li, I. Rashkov, J. Espartero, N. Manolova and M. Vert, Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with long poly(L-lactic acid) blocks, Macromolecules, 1996, 29, 57–62 CrossRef CAS.
- K. Kim, S. Chung, I. Chin, M. Kim and J. Yoon, Crystallization behavior of biodegradable amphiphilic poly(ethylene glycol)-poly(L-lactide) block copolymers, J. Appl. Polym. Sci., 1999, 72, 341–348 CrossRef CAS.
- D. Kubies, F. Rypácek, J. Kovárová and F. Lednický, Micro-domain structure in polylactide-block-poly(ethylene oxide) copolymer films, Biomaterials, 2000, 21, 529–536 CrossRef CAS.
- G. Maglio, A. Migliozzi and R. Palumbo, Thermal properties of di- and triblock copolymers of poly(L-lactide) with poly(oxyethylene) or poly(e-caprolactone), Polymer, 2003, 44, 369–375 CrossRef CAS.
- C. Cai, L. Wang and C. M. Dong, Synthesis, characterization, effect of architecture on crystallization, and spherulitic growth of poly(L-lactide)-b-poly(ethylene oxide) copolymers with different branch arms, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2034–2244 CrossRef CAS.
- C. I. Huang, S. H. Tsai and C. M. Chen, Isothermal crystallization behavior of poly(l-lactide) in poly(l-lactide)-block-poly(ethylene glycol) diblock copolymers, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 2438–2448 CrossRef CAS.
- T. Wu, Y. He, Z. Fan, J. Wei and S. Li, Investigations on the morphology and melt crystallization of poly(L-lactide)-poly(ethylene glycol) diblock copolymers, Polym. Eng. Sci., 2008, 48, 425–433 CAS.
- S. Y. Huang, H. F. Li, S. C. Jiang, X. S. Chen and L. J. An, Morphologies and structures in poly(L-lactide-b-ethylene oxide) copolymers determined by crystallization, microphase separation, and vitrification, Polym. Bull., 2011, 67, 885–902 CrossRef CAS PubMed.
- Z. Gan, B. Jiang and J. Zhang, Poly(ε-caprolactone)/poly(ethylene oxide) diblock copolymer. I: Isothermal crystallization and melting behavior, J. Appl. Polym. Sci., 1996, 59, 961–967 CrossRef CAS.
- Z. Gan, J. Zhang and B. Jiang, Poly(ε-caprolactone)/poly(ethylene oxide) diblock copolymer. II: Nonisothermal crystallization and melting behavior, J. Appl. Polym. Sci., 1997, 63, 1793–1804 CrossRef CAS.
- Y. K. Choi, Y. H. Bae and S. W. Kim, Star-shaped poly(ether-ester) block copolymers: synthesis, characterization, and their physical properties, Macromolecules, 1998, 31, 8766–8774 CrossRef CAS.
- B. Bogdanov, A. Vidts, E. Schacht and H. Berghmans, Isothermal crystallization of poly(ε-caprolactone-ethylene glycol) block copolymers, Macromolecules, 1999, 32, 726–731 CrossRef CAS.
- C. Lu, S. R. Guo, Y. Zhang and M. Yin, Synthesis and aggregation behavior of four types of different shaped PCL–PEG block copolymers, Polym. Int., 2006, 55, 694–700 CrossRef CAS.
- H. Takeshita, K. Fukumoto, T. Ohnishi, T. Ohkubo, M. Miya and K. Takenaka, Formation of lamellar structure by competition in crystallization of both components for crystalline–crystalline block copolymers, Polymer, 2006, 47, 8210–8218 CrossRef CAS PubMed.
- L. Li, F. Meng, Z. Zhong, D. Byelov, W. H. de Jeu and J. Feijen, Morphology of a highly asymmetric double crystallizable poly(ε-caprolactone-b-ethylene oxide) block copolymer, J. Chem. Phys., 2007, 126, 024904 CrossRef PubMed.
- R. V. Castillo, A. J. Müller, J. M. Raquez and P. Dubois, Crystallization Kinetics and Morphology of Biodegradable Double Crystalline PLLA-b-PCL Diblock Copolymers, Macromolecules, 2010, 43, 4149–4160 CrossRef CAS.
- R. M. Ho, P. Hsieh, W. Tseng, C. Lin, B. Huang and B. Lotz, Crystallization-induced orientation for microstructures of poly(L-lactide)-b-poly(ε-caprolactone) diblock copolymers, Macromolecules, 2003, 36, 9085–9092 CrossRef CAS.
- I. Hamley, P. Parras, V. Castelletto, R. Castillo, A. J. Müller and E. Pollet, et al. Melt structure and its transformation by sequential crystallization of the two blocks within poly(L-lactide)-block-Poly(ε-caprolactone) double crystalline diblock copolymers, Macromol. Chem. Phys., 2006, 207, 941–953 CrossRef CAS.
- B. Bogdanov, A. Vidts, A. Van Den Bulcke, R. Verbeeck and E. Schacht, Synthesis and thermal properties of poly(ethylene glycol)-poly(-caprolactone) copolymers, Polymer, 1998, 39, 1631–1636 CrossRef CAS.
- L. Piao, Z. Dai, M. Deng, X. Chen and X. Jing, Synthesis and characterization of PCL/PEG/PCL triblock copolymers by using calcium catalyst, Polymer, 2003, 44, 2025–2031 CrossRef CAS.
- P. Ghoroghchian, G. Li, D. Levine, K. Davis, F. Bates and D. Hamer, et al., Bioresorbable vesicles formed through spontaneous self-assembly of amphiphilic poly(ethylene oxide)-block-polycaprolactone, Macromolecules, 2006, 39, 1673–1675 CrossRef CAS PubMed.
- Y. L. Loo, R. A. Register, A. J. Ryan and G. T. Dee, Polymer crystallization confined in one, two, or three dimensions, Macromolecules, 2001, 34, 8968–8977 CrossRef CAS.
- J. Yang, T. Zhao, Y. Zhou, L. Liu, G. Li, E. Zhou and X. Chen, Single Crystals of the Poly(L-lactide) Block and the Poly(ethylene glycol) Block in Poly(L-lactide) Poly(ethylene glycol) Diblock Copolymer, Macromolecules, 2007, 40, 2791–2797 CrossRef CAS.
- J. Yang, Y. Liang, J. Luo, C. Zhao and C. C. Han, Multilength Scale Studies of the Confined Crystallization in Poly(L-lactide)-block-Poly(ethylene glycol) Copolymer, Macromolecules, 2012, 45, 4254–4261 CrossRef CAS.
- Y. Kikkawa, H. Abe, T. Iwata, Y. Inoue and Y. Doi, Crystallization, stability, and enzymatic degradation of poly(L-lactide) thin film, Biomacromolecules, 2002, 3, 350–356 CrossRef CAS PubMed.
- S. Nurkhamidah and E. M. Woo, Phase-Separation-Induced Single-Crystal Morphology in Poly(L-lactic acid) Blended with Poly(1,4-butylene adipate) at Specific Composition, J. Phys. Chem. B, 2011, 115, 13127–13138 CrossRef CAS PubMed.
- I. W. Hamley, The physics of block copolymers, Oxford university press, Oxford, 1998 Search PubMed.
- J. Kim, D. Park, M. Lee and K. Ihn, Synthesis and crystallization behavior of poly(L-lactide)-block-poly(ε-caprolactone) copolymer, Polymer, 2001, 42, 7429–7441 CrossRef CAS.
- L. Zhu, B. H. Calhoun, Q. Ge, R. P. Quirk, S. Z. D. Cheng and E. L. Thomas, et al., Initial-stage growth controlled crystal orientation in nanoconfined lamellae of a sell-assembled crystalline-amorphous diblock copolymer, Macromolecules, 2001, 34, 1244–1251 CrossRef CAS.
- R. M. Ho, P. Y. Hsieh, W. H. Tseng, C. C. Lin, B. H. Huang and B. Lotz, Crystallization-induced orientation for microstructure of poly(L-lactide)-b-poly(ε-caprolactone) diblock copolymers, Macromolecules, 2003, 36, 9085–9092 CrossRef CAS.
- C. De Rosa, C. Park, B. Lotz, J. Wittmann, L. Fetters and E. Thomas, Control of molecular and microdomain orientation in a semicrystalline block copolymer thin film by epitaxy, Macromolecules, 2000, 33, 4871–4876 CrossRef CAS.
- C. Park, C. De Rosa and E. Thomas, Large area orientation of block copolymer microdomains in thin films via directional crystallization of a solvent, Macromolecules, 2001, 34, 2602–2606 CrossRef CAS.
- Y. Kikkawa, H. Abe, T. Iwata, Y. Inoue and Y. Doi, In situ observation of crystal growth for poly[(S)-lactide] by temperature-controlled atomic force microscopy, Biomacromolecules, 2001, 2, 940–945 CrossRef CAS.
- L. Liu, B. Z. Jiang and E. L. Zhou, Study of semicrystalline-amorphous diblock copolymers. 1. Microphase separation, glass transition and crystallization of tetrahydrofuran methyl methacrylate diblock copolymers, Polymer, 1996, 37, 3937–3943 CrossRef CAS.
- D. Quiram, R. Register and G. Marchand, Crystallization of asymmetric diblock copolymers from microphase-separated melts, Macromolecules, 1997, 30, 4551–4558 CrossRef CAS.
- D. Quiram, R. Register, G. Marchand and A. Ryan, Dynamics of structure formation and crystallization in asymmetric diblock copolymers, Macromolecules, 1997, 30, 8338–8343 CrossRef CAS.
- I. W. Hamley, J. Fairclough, F. Bates and A. Ryan, Crystallization thermodynamics and kinetics in semicrystalline diblock copolymers, Polymer, 1998, 39, 1429–1437 CrossRef CAS.
- T. Shiomi, H. Tsukada, H. Takeshita, K. Takenaka and Y. Tezuka, Crystallization of semicrystalline block copolymers containing a glassy amorphous component, Polymer, 2001, 42, 4997–5004 CrossRef CAS.
- S. Nojima, K. Hashizume, A. Rohadi and S. Sasaki, Crystallization of epsilon-caprolactone blocks within a crosslinked microdomain structure of poly(epsilon-caprolactone)-block-polybutadiene, Polymer, 1997, 38, 2711–2718 CrossRef CAS.
- A. Kelarakis, S. M. Mai, C. Booth and A. J. Ryan, Can rheometry measure crystallization kinetics: A comparative study using block copolymers, Polymer, 2005, 46, 2739–2747 CrossRef CAS PubMed.
- S. Nojima, D. Inokawa, T. Kawamura and K. Nitta, Dynamic mechanical study of block copolymer crystallization confined within spherical nanodomains, Polym. J., 2008, 40, 986–991 CrossRef CAS.
- J. Xu, V. Bellas, B. Jungnickel, B. Stuhn and M. Rehahn, Equilibrium melting temperature of poly(ferrocenyl dimethylsilane) in homopolymers and lamellar diblock copolymers with polystyrene, Macromol. Chem. Phys., 2010, 211, 1261–1271 CrossRef CAS.
- F. F. Xue, X. S. Chen, L. J. An, S. S. Funari and S. C. Jiang, Soft nanoconfinement effects on the crystallization behavior of asymmetric poly(ethylene oxide)-block-poly(ε-caprolactone) diblock copolymers, Polym. Int., 2012, 61, 909–917 CrossRef CAS.
- S. C. Jiang, C. L. He, Y. F. Men, X. Chen, L. J. An, S. S. Funari and C. M. Chan, Study of temperature dependence of crystallisation transitions of a symmetric PEO-PCL diblock copolymer using simultaneous SAXS and WAXS measurements with synchrotron radiation, Eur. Phys. J. E: Soft Matter Biol. Phys., 2008, 27, 357–364 CrossRef CAS PubMed.
- E. Núńez and U. W. Gedde, Single crystal morphology of star-branched polyesters with crystallisable poly(ε-caprolactone) arms, Polymer, 2005, 46, 5992–6000 CrossRef PubMed.
- V. H. Mareau and R. E. Prud'homme, In situ hot stage atomic force microscopy study of poly(ε-caprolactone) crystal growth in ultrathin films, Macromolecules, 2005, 38, 398–408 CrossRef CAS.
- H. Tsuji, M. Hosokawa and Y. Sakamoto, Ternary stereocomplex formation of one L-configured and two D-configured optically active polyesters, poly(L-2-hydroxybutanoic acid), poly(D-2-hydroxybutanoic acid), and poly(D-lactic acid), ACS Macro Lett., 2012, 1, 687–691 CrossRef CAS.
- H. Tsuji, M. Nakano, M. Hashimoto, K. Takashima and S. Katsura, Mizuno A. Electrospinning of Poly(lactic acid) Stereocomplex Nanofibers, Biomacromolecules, 2006, 7, 3316–3320 CrossRef CAS PubMed.
- W. H. Watanabe, C. F. Ryan, P. C. Fleischer Jr and B. S. Garrett, Measurement of the tacticity of syndiotactic poly-(methyl methacrylate) by the gel melting point, J. Phys. Chem., 1961, 65, 896 CrossRef CAS.
- M. T. Casas, J. Puiggali, J. M. Raquez, P. Dubois, M. E. Córdova and A. J. Müller, Single crystals morphology of biodegradable double crystalline PLLA-b-PCL diblock copolymers, Polymer, 2011, 52, 5166–5177 CrossRef CAS PubMed.
- T. Fukuzawa, I. Uematsu and Y. Uematsu, Physical properties of mixtures of poly(γ-benzyl-L-glutamate) and poly(γ-benzyl-D-glutamte), Polym. J., 1974, 6, 537–541 CrossRef CAS.
- N. Matawhima, K. Hikichi, A. Tsutsumi and M. Kaneko, X-ray scattering of synthetic poly(α-amino acid)s in the solid state. II. Phase transition of DL mixtures of poly(γ-benzyl glutamate)s, Polym. J., 1975, 3, 382–386 Search PubMed.
- Y. Baba and A. Kagemoto, Heats of dissociation of mixture of poly(γ-benzyl L- and D-glutamate), Macromolecules, 1977, 10, 458–460 CrossRef CAS.
- Y. Ikada, K. Jamshidi, H. Tsuji and S.-H. Hyon, Stereocomplex formation between enantiomeric poly(lactides), Macromolecules, 1987, 20, 904–906 CrossRef CAS.
- N. Yui, P. J. Dijkstra and J. Feijen, Stereo block copolymers of L-and D-lactides, Makromol. Chem., 1990, 191, 481–488 CrossRef CAS.
- N. Spassky, M. Wisniewski, C. Pluta and A. Le Borgne, Highly stereoelective polymerization of rac-(D,L)-lactide with a chiral Schiff's base/aluminium alkoxide initiator, Macromol. Chem. Phys., 1996, 197, 2627–2637 CrossRef CAS.
- K. Fukushima, Y. Furuhashi, K. Sogo, S. Miura and Y. Kimura, Stereoblock poly(lactic acid):synthesis via solid-state polycondensation of a stereocomplexed mixture of poly(L-lactic acid) and poly(D-lactic acid), Macromol. Biosci., 2005, 5, 21–29 CrossRef CAS PubMed.
- K. Fukushima, M. Hirata and Y. Kimura, Synthesis and characterization of stereoblock poly(lactic acid)s with nonequivalent D/L sequence ratios, Macromolecules, 2007, 40, 3049–3055 CrossRef CAS.
- M. Hirata and Y. Kimura, Thermomechanical properties of stereoblock poly(lactic acid)s with different PLLA/PDLA block compositions, Polymer, 2008, 49(11), 2656–2661 CrossRef CAS PubMed.
- M. Kakuta, M. Hirata and Y. Kimura, Stereoblock Polylactides as High-Performance Bio-Based Polymers, J. Macromolecular Sci. R, Part C: Polymer Reviews, 2009, 49, 107–140 CAS.
- P. De Santis and A. J. Kovacs, Molecular conformation of poly(S-lactic acid), Biopolymers, 1968, 6, 299–306 CrossRef CAS PubMed.
- W. Hoogsten, A. R. Postema, A. J. Pennings, G. ten Brinke and P. Zugenmair, Crystal structure, conformation, and morphology of solution-spun poly(L-lactide) fibers, Macromolecules, 1990, 23, 634–642 CrossRef.
- J. Kobayashi, T. Asahi, M. Ichiki, A. Oikawa, H. Suzuki, T. Watanabe, E. Fukada and Y. Shikinami, Structural and optical properties of poly lactic acids, J. Appl. Phys., 1995, 77, 2957–2973 CrossRef CAS PubMed.
- T. Miyata and T. Masuko, Morphology of poly(L-lactide) solution-grown crystals, Polymer, 1997, 38, 4003–4009 CrossRef CAS.
- T. Okihara, M. Tsuji, A. Kawaguchi, K. Katayama, H. Tsuji, S.-H. Hyon and Y. Ikada, Crystal structure of stereocomplex of poly(L-lactide) and poly(D-lactide), J. Macromol. Sci., Part B: Phys., 1991, 30, 119–140 CrossRef CAS.
- H. Tsuji and Y. Ikada, Stereocomplex formation between enantiomeric poly(lactic acid)s. Stereocomplexation from the melt, Macromolecules, 1993, 26, 6918–6926 CrossRef CAS.
- H. Tsuji, Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications, Macromol. Biosci., 2005, 5, 569–597 CrossRef CAS PubMed.
- M. H. Rahaman and H. Tsuji, Isothermal Crystallization and Spherulite Growth Behavior of Stereo Multiblock Poly(lactic acid)s: Effects of Block Length, J. Appl. Polym. Sci., 2013, 129, 2502–2517 CrossRef CAS.
- H. Uehara, Y. Karaki, S. Wada and T. Yamanobe, Stereo-complex crystallization of poly(lactic acid)s in block-copolymer phase separation, ACS Appl. Mater. Interfaces, 2010, 2(10), 2707–2710 CAS.
- M. H. Rahaman and H. Tsuji, Isothermal crystallization and spherulite growth behavior of stereo multiblock poly(lactic acid)s: effects of block length, J. Appl. Polym. Sci., 2013, 129, 2502–2517 CrossRef CAS.
- C. L. Huang, L. Jiang, J. Zhang, J. Zeng, K. Yang and Y. Z. Wang, Poly(butylene succinate)-poly(ethylene glycol) multiblock copolymer: synthesis, structure, properties and shape memory performance, Polym. Chem., 2012, 3, 800–808 RSC.
- J. Zeng, Q. Zhu, X. Lu, Y. He and Y. Z. Wang, From miscible to partially miscible biodegradable double crystalline poly(ethylene succinate)-b-poly(butylene succinate) multiblock copolymers, Polym. Chem., 2012, 3, 399–408 RSC.
- C. L. Huang, L. Jiao, J. B. Zeng, M. Zhang, L. P. Xiao, K. K. Yang and Y. Z. Wang, Crystallization behavior and morphology of double crystalline poly(butylene succinate)-poly(ethylene glycol) multiblock copolymers, Polymer, 2012, 53, 3780–3790 CrossRef CAS PubMed.
- Z. Zhang, J. Ren, Y. Feng, J. Li and W. Z. Yuan, Microwave-Assisted Synthesis of Star-Shaped Poly(e-caprolactone)-block-poly(L-lactide) Copolymers and the Crystalline Morphologies, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5063–5071 CrossRef CAS.
- C. M. Dong, K. Y. Qiu, Z. W. Gu and X. D. Feng, Poly(ε-caprolactone)-b-(D,L-lactic acid-alt-glycolic acid) with Multifunctional Initiator and SnOct2 Catalyst, Macromolecules, 2001, 21, 4691–4696 CrossRef.
- H. Jin, W. Huang, X. Zhu, Y. F. Zhou and D. Y. Yan, Biocompatible or biodegradable hyperbranched polymers: from self-assembly to cytomimetic applications, Chem. Soc. Rev., 2012, 41, 5986–5997 RSC.
- W. Z. Yuan, J. Zhang, H. Zou and J. Ren, Synthesis, crystalline morphologies, self-assembly, and properties of H-shaped amphiphilic dually responsive terpolymers, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2541–2552 CrossRef CAS.
- I. W. Hamley, Ordering in thin films of block copolymers: fundamentals to potential applications, Prog. Polym. Sci., 2009, 34, 1161–1210 CrossRef CAS PubMed.
- R. A. Segalman, Patterning with block copolymer thin films, Mater. Sci. Eng., R, 2005, 48, 191–226 CrossRef PubMed.
- M. Ramanathan and S. B. Darling, Mesoscale morphologies in polymer thin films, Prog. Polym. Sci., 2011, 36, 793–812 CrossRef CAS PubMed.
- F. J. Zhang, Y. Z. Chen, H. Y. Huang, Z. J. Hu and T. B. He, Boundary effect of relief structure on crystallization of diblock copolymer in thin films, Langmuir, 2003, 19, 5563–5566 CrossRef CAS.
- F. J. Zhang, H. Y. Huang, Z. J. Hu, Y. Z. Chen and T. B. He, Crystallization of weakly segregated poly(styrene-b-epsilon-caprolactone) diblock copolymer in thin films, Langmuir, 2003, 19, 10100–10108 CrossRef CAS.
- J. Fu, Y. Cong, J. Li, B. Luan, C. Y. Pan, Y. M. Yang, B. Y. Li and Y. Han, Hole nucleation and growth induced by crystallization and microphase separation of thin semicrystalline diblock copolymer films, Macromolecules, 2004, 37, 6918–6925 CrossRef CAS.
- Y. Li, Y. L. Loo, R. A. Register and P. F. Green, Influence of interfacial constraints on the morphology of asymmetric crystalline-amorphous diblock copolymer films, Macromolecules, 2005, 38, 7745–7753 CrossRef CAS.
- W. H. Huang, C. X. Luo, J. L. Zhang and Y. C. Han, Formation of ordered microphase-separated pattern during spin coating of ABC triblock copolymer, J. Chem. Phys., 2007, 126, 104901 CrossRef PubMed.
- S. Y. Huang, H. F. Li, Y. R. Shang, D. H. Yu, G. Li, S. C. Jiang, X. S. Chen and L. J. An, Chloroform micro-evaporation induced ordered structures of poly(L-lactide) thin films, RSC Adv., 2013, 3, 13705–13711 RSC.
- S. Y. Huang, H. F. Li, H. Y. Wen, D. H. Yu, S. C. Jiang, G. Li, X. S. Chen and L. J. An, Solvent micro-evaporation and concentration gradient synergistically induced crystallization of poly(L-lactide) and ring banded supra-structures with radial periodic variation of thickness, CrystEngComm, 2014, 16, 94–101 RSC.
- J. C. Zhao, J. M. Zhang, X. L. Duan, Z. Peng and S. H. Wang, Formation mechanism, chain folding, and growth behavior of the intriguing fiber-like crystal of poly (ethylene oxide-b-epsilon-caprolactone) block copolymer in ultrathin films, Polymer, 2011, 52, 2085–2093 CrossRef CAS PubMed.
- T. Vilgis and A. Halperin, Aggregation of coil crystalline block copolymers-equilibrium crystallization, Macromolecules, 1991, 24, 2090–2095 CrossRef CAS.
- L. Cao, I. Manners and M. A. Winnik, Influence of the interplay of crystallization and chain stretching on micellar morphologies solution self-assembly of coil-crystalline poly(isoprene-block ferrocenylsilane), Macromolecules, 2002, 35, 8258–8260 CrossRef CAS.
- N. Kang, M. É. Perron, R. E. Prud'homme, Y. B. Zhang, G. Gaucher and G. C. Leroux, Stereocomplex Block Copolymer Micelles: Core–Shell Nanostructures with Enhanced Stability, Nano Lett., 2005, 5(2), 315–319 CrossRef CAS PubMed.
- M. Spasova, N. Manolova, D. Paneva, R. Mincheva, P. Dubois, I. Rashkov, V. Maximova and D. Danchev, Polylactide Stereocomplex-Based Electrospun Materials Possessing Surface with Antibacterial and Hemostatic Properties, Biomacromolecules, 2010, 11, 151–159 CrossRef CAS PubMed.
- E. Huitron-Rattinger, K. Ishida, A. Romo-Uribe and P. T. Mather, Thermally modulated nanostructure of poly(ε-caprolactone)Eposs multiblock thermoplastic polyurethane, Polymer, 2013, 54, 3350–3362 CrossRef CAS PubMed.
- X. H. Wu, S. Li, F. Coumes, V. Darcos, J. L. K. Him and P. Bron, Modeling and self-assembly behavior of PEG–PLA–PEG triblock copolymers in aqueous solution, Nanoscale, 2013, 5, 9010–9017 RSC.
- J. Zhang, L. Q. Wang, H. J. Wang and K. H. Tu, Micellization phenomena of aphiphilic block copolymers based on methoxy poly(ethylene glycol) and either crystalline or amorphous poly(caprolactone-b-lactide), Biomacromolecules, 2006, 7, 2492–2500 CrossRef CAS PubMed.
- S. Payyappilly, S. Dhara and S. Chattopadhyay, Thermoresponsive biodegradable PEG–PCL–PEG based injectable hydrogel for pulsatile insulin delivery, J. Biomed. Mater. Res., Part A, 2014, 102, 1500–1509 CrossRef PubMed.
- H. Wang, J. He, M. Z. Zhang, Y. F. Tao, F. Li, K. C. Tamb and P. H. Ni, Biocompatible and acid-cleavable poly(ε-caprolactone)-acetal-poly(ethylene glycol)-acetal-poly(ε-caprolactone) triblock copolymers: synthesis, characterization and pH-triggered doxorubicin delivery, J. Mater. Chem. B, 2013, 1, 6596–6607 RSC.
- K. Y. Peng, S. W. Wang, M. Y. Hua and R. Lee, Amphiphilic photocleavable block copolymers based on monomethyl poly(ethylene glycol) and poly(4-substituted-ε-caprolactone): synthesis, characterization, and cellular uptake, RSC Adv., 2013, 3, 18453–18463 RSC.
- K. P. McNamee, L. M. Pitet and D. M. Knauss, Synthesis, assembly, and cross-linking of polymer amphiphiles in situ: polyurethane–polylactide core–shell particles, Polym. Chem., 2013, 4, 2546–2555 RSC.
- M. Danquah, T. Fujiwara and R. I. Mahato, Self-assembling methoxy poly(ethylene glycol)-b-poly(carbonate-co-L-lactide) block copolymers for drug delivery, Biomaterials, 2010, 31, 2358–2370 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |