Retracted Article: Light-triggered nitric oxide release and targeted fluorescence imaging in tumor cells developed from folic acid-graft-carboxymethyl chitosan nanospheres†
Rijun Guiab,
Ajun Wan*ab,
Yalei Zhanga,
Huili Li*c and
Tingting Zhaob
aState Key Laboratory of Pollution Control and Resources Reuse, National Engineering Research Center of Facilities Agriculture, Tongji University, Shanghai 200092, P.R. China. E-mail: wanajun@tongji.edu.cn; Fax: +86 21 54745706; Tel: +86 21 34201245
bDepartment of Chemistry, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P.R. China
cSchool of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, P.R. China. E-mail: lihl@sjtu.edu.cn
First published on 29th May 2014
Abstract
Folic acid (FA) grafted carboxymethyl chitosan (CMC) nanospheres (CMC–FA) were prepared via a facile carboxy-amine coupling reaction, and were further developed toward light-triggered nitric oxide (NO) release and targeted fluorescence imaging in cells. Under electrostatic adsorption interactions, photosensitive NO donors (Fe4S3(NO)7−, RBS) and polyethleneimine-stabilized carbon quantum dots (CQD) were respectively loaded into CMC–FA to form CMC–FA–RBS and CMC–FA–CQD hybrid nanospheres. These as-prepared nanospheres exhibited high colloidal stabilities in phosphate buffered saline (pH 4.9–9.2) and bovine serum albumin (0–48 h incubation), and produced negligible cytotoxicity. NO was released from CMC–FA–RBS nanospheres upon light excitation (365 nm), demonstrating a smart “on–off” switch for controllable NO release. Targeted fluorescence imaging of CMC–FA–CQD nanospheres was investigated and confirmed in folate receptor (FR) over-expressed tumor (HeLa) cells, compared to other cells without or with relatively low expression of FR.
1 Introduction
Carboxymethyl chitosan (CMC), as one of the most important water-soluble chitosan derivatives, contains –COOH and –NH2 groups in the molecule,1 and exhibits a variety of potential applications especially as a promising biomaterial.2 In view of its attractive physical, chemical and biological properties such as low toxicity and good biocompatibility, CMC has been extensively utilized in the biomedical field.3–6 Folic acid (FA) is a high-affinity ligand to folate receptor (FR) and is not produced endogenously. As established, a cell membrane-associated FR, i.e. FA-binding protein with a natural glycol-polypeptide (∼38 kDa), can be overexpressed by many epithelial-derived tumors, including mammary gland, lung, kidney, testis, prostate, but minimally expressed in normal tissues.7–9 The FA molecule has two –COOH groups, termed α- and γ- (Scheme 1), and the latter exhibits a much higher reactivity in a carbodiimide-mediated coupling to amino groups.10 FA linked with other substances via its γ-COOH groups still maintains a strong affinity to its receptor (FR).11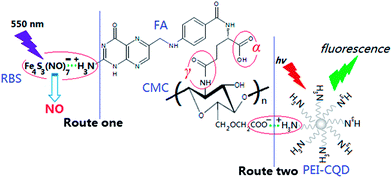 | ||
| Scheme 1 Schematic representation of CMC-graft-FA conjugates and the formation routes of NO release and fluorescence emission hybrid systems. | ||
Commonly, the combination of CMC and FA via physical or chemical routes is expected because the resulting CMC–FA conjugates can be developed as a biocompatible drug delivery system (DDS) or carrier for targeted drug delivery and controlled drug release in tumor tissues. For instance, Jayakumar et al. prepared FA conjugated CMC that was coordinated to Mn-doped ZnS quantum dots (QDs), and developed the FA–CMC–ZnS–Mn nanoparticles (NPs) for targeting, controllable drug (2,4-dihydroxy-5-fluorpyrimidin, 5-FU) delivery and imaging of cancer cells (L929/MCF-7).12 Utilizing CMC as coupling agents, Bhattacharya et al. designed amine functionalized magneto-fluorescent NPs from covalent immobilization of rhodamine isothiocyanate on the amine-functionalized surface for folate receptor targeted bimodal imaging.13 Zhang et al. synthesized FA conjugated CMC that loaded with magnetic Fe3O4 NPs and CdTe QDs, and studied the organic–inorganic hybrids' applications for targeted drug (adriamycin, ADM) delivery and cell (L02/HepG2) imaging.2 However, using the QDs containing noble metal ions (e.g. Zn2+ and Cd2+) in CMC–FA systems for intracellular imaging is questionable because of their potential cytotoxicity.14 Compared to traditional semiconductor QDs and organic dyes,13 photoluminescence (PL) carbon quantum dots (CQD) are superior in terms of ultralow toxicity and excellent biocompatibility, which have been further exploited toward CQD's significant applications in biological labeling, bio-imaging and drug delivery.15–18 Provided that QDs in CMC–FA systems (the two cases referred above) are replaced by CQD, in vitro/in vivo cell imaging of CQD-loaded CMC–FA conjugates becomes more feasible in clinical manipulation.
Nitric oxide (NO), as a diatomic free radical produced in human body, regulates several biological functions in cardiovascular, respiratory and nervous systems.19,20 Usually, NO is actively involved in immune system response, and it mediates macrophage destruction from foreign pathogens.21 Owing to the complex and extensive actions of NO in physiological environments, to develop effective NO donors for storing and releasing NO in a controllable manner is still an urgent and challenged task. In comparison with spontaneous release of NO from diazeniumdiolate NO donors under physiological conditions (37 °C, pH 7.4),22–24 light-triggered NO release from photosensitive NO donors (e.g. Fe4S3(NO)7−, RBS Na+ salt,25 Part S1 in ESI†) is much more promising due to the highly controllable release in aqueous systems.26,27 To the best of our knowledge, there is no report referring to CMC–FA-based DDS for light-triggered controlled release of NO. Provided that RBS is introduced into CMC–FA systems, the new DDS combining biocompatibility, targeting of tumor cells and light-controlled release of NO into one unit, would exhibit significant potentials in biological and biomedical fields.
In this article, FA-graft-CMC conjugates (i.e. CMC–FA nanospheres) were prepared via a simple carboxy-amine coupling reaction between the –COOH of FA and –NH2 of CMC in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and dimethyl sulfoxide (DMSO). The as-prepared CMC–FA nanospheres were further developed by introducing RBS (as the NO precursor) and polyethleneimine (PEI)-stabilized CQD (as the PL probe) into CMC–FA networks under electrostatic adsorption interactions. The resulting CMC–FA–RBS and CMC–FA–CQD hybrid nanospheres would enable effective light-triggered release of NO and targeted PL imaging in tumor cells with over-expression of FR. (Scheme 1). A whole set of physiochemical characteristics of CMC–FA, CMC–FA–RBS and CMC–FA–CQD were performed. Stability and cytotoxicity of these nanospheres were studied. Especially, the light-triggered NO release of CMC–FA–RBS and targeted PL imaging of CMC–FA–CQD in human cells without or with different degrees of FR-expression were regularly investigated.
2 Materials and methods
2.1 Materials
CMC (Mw 400 kDa, the degrees of deacetylation and carboxymethylation are 90% and 1.112, respectively), EDC, DMSO and FA were purchased from Sinopharm Chemical Reagent Co. (China). [Fe4S3(NO)7]− Roussin's Black Salt (RBS) was prepared according to the work from Seyferth et al.25 The RBS in the solid form is stored in the dark and inert atmosphere. Glucose, PEI and conventional chemicals with analytical grade were from Shanghai Chemical Reagent Co. (China). 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay, bovine serum albumin (BSA, 66.43 kDa) and other biological reagents were brought from Invitrogen Co. (USA). L02, A549, HeLa and HepG2 cells were provided by the cell bank of Shanghai Science Academe (China). All reagents can be utilized directly as received without any purification. The water used in experiments was prepared using a Milli-Q water purification system (Milli-Pore, Bedford, USA). The phosphate buffer saline (PBS) was made up of NaCl, KCl, Na2HPO4 and KH2PO4 with an appropriate mol ratio in water and adjusted to a desired pH for direct use. PEI-stabilized QDs and aqueous dispersed liquid (1 wt%) were prepared firstly, and the detailed procedures are available (Part S1 in ESI†).2.2 Characterization
1H-NMR spectrogram was performed by 1H-nuclear magnetic resonance spectrometer (AMX-500, Bruker) using D2O as the solvent. Fourier transform infrared spectra (FTIR, Nicolet, USA) were recorded with a Nicolet 6700 FTIR spectrometer, and the scanning region is from 500 to 4000 cm−1. Transmission electron microscope (TEM, Jeol, Japan) images were acquired with a JEOL JEM-1400 TEM operating at 120 kV of acceleration voltage. Scanning electron microscopy (SEM, JEOL, Japan) images were obtained with a JCM-5700 SEM. UV-vis absorption spectra were recorded with a UV-2450 spectrophotometer (Shimadzu, Japan). PL emission spectra were measured with a FLSP 920 fluorescence spectrophotometer (Edinburgh Instruments, Britain). Images of cells were obtained by a FV-300 IX71 confocal laser fluorescence microscope (CLFM, Olympus).2.3 Synthesis of CMC–FA nanospheres
At room temperature (20 °C), a 10 mL solution of EDC·HCl and FA in anhydrous DMSO was prepared by rapidly stirring until FA was wholly dissolved (∼1 h). It was added into an aqueous solution of CMC (1 wt%). The pH of the mixed solution was regulated to 4.7 by adding acetic acid, and then stirred in the dark for 16 h. The reaction solution was adjusted to pH 9.0 by dropwise addition of aqueous sodium hydroxide (1 M), and then dialyzed (Spectra/Por 7 dialysis membrane, MWCO 5 × 104) against water frequently, which lasted for 7 days. The resultant product (i.e. CMC–FA nanospheres) was collected by lyophilization. Dried product was used for characterization or re-dispersed in PBS (10 mM, pH 7.4) for subsequent experiments.2.4 Synthesis of CMC–FA–RBS and CMC–FA–CQD hybrid nanospheres
Typically, CMC–FA nanospheres were dispersed in PBS (10 mM, pH 7.4), and their concentration was adjusted to be 1 mg mL−1. Under slight stirring and ultrasonication, 10 mL aqueous solution of RBS or PEI–CQD (1 wt%) was dropwise injected into 10 mL CMC–FA solution to obtain homogeneous PBS of CMC–FA–RBS and CMC–FA–CQD nanospheres, respectively. Finally, these nanospheres were purified by using the same procedure as referred in the synthesis of CMC–FA nanospheres.2.5 Measurements of stability and cytotoxicity
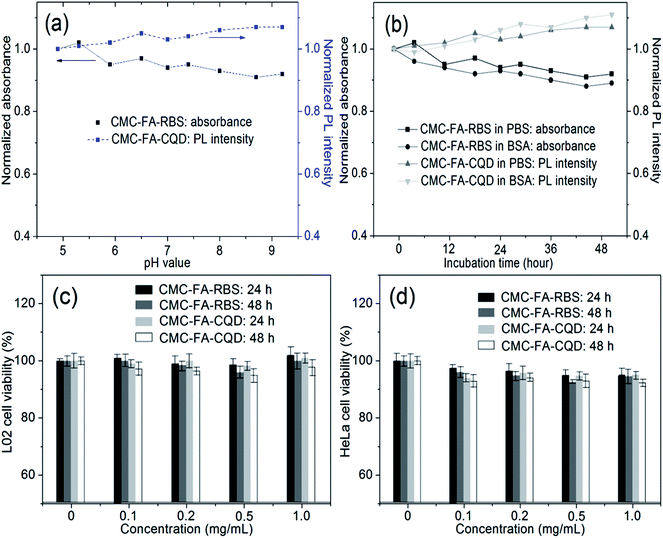
To estimate the colloidal stability of hybrid nanospheres, 1 mg mL−1 of CMC–FA–RBS (or CMC–FA–CQD) were incubated in 10 mM of PBS with different pH values (4.9, 5.3, 6.0, 6.5, 7.0, 7.4, 8.0, 8.7, and 9.2). Then, the absorbance (at 375 nm) of CMC–FA–RBS, and PL intensities (at 510 nm) of CMC–FA–CQD were recorded after 10 min incubation in the dark. As a reference, 1 mg mL−1 of CMC–FA–RBS (or CMC–FA–CQD) were incubated in PBS (10 mM, pH 7.4) and BSA (10 mM), respectively. Then, the absorbance of CMC–FA–RBS, and PL intensities of CMC–FA–CQD were recorded at different incubation times (12, 24, 36, and 48 h). To test the cytotoxicity of hybrid nanospheres, L02 cells and HeLa cells were cultured as subconfluent monolayers on 25 cm2 cell culture plates with vent caps in 1 × minimum essential α medium supplemented with fetal bovine serum (10%) in a humidified incubator at 37 °C containing CO2 (5%). When these cells had grown to subconfluence, they were dissociated from the surface with a solution of trypsin (0.25%) plus EDTA for 30 s, and aliquots of mixture solution (100 μL) were seeded (1 × 104 cells) into a 96-well plate. After 24 h incubation at 37 °C, the medium was replaced with 100 μL of serum-free DMEM medium containing 0.1, 0.2, 0.5, and 1.0 mg mL−1 of CMC–FA–RBS (or CMC–FA–CQD). These treated cells were incubated for 24 and 48 h at 37 °C. The cells treated with alone medium were used for cell death controls. Cell viabilities were quantitated using a standard MTT assay.2.6 NO release experiments from CMC–FA–RBS
To investigate the light-triggered release of NO developed from CMC–FA–RBS, 1.0 mg mL−1 of CMC–FA–RBS were incubated in 10 mM of PBS (pH 7.4) and continuously excited by a laser (365 nm). The concentration of released NO in aqueous solution was calculated using the method of colorimetric Griess reaction (Part S2 in ESI†). NO concentrations were recorded at various excitation times (0–10 min). The concentrations of NO released under commutative excitation (1 min) and storing in the dark (1 min) were recorded regularly. NO concentrations from CMC–FA–RBS in the dark were recorded after different incubation times (0–10 min). As a reference, the NO concentrations from CMC–FA nanospheres were recorded at different excitation times (0–10 min).2.7 Cellular uptake and imaging from CMC–FA–CQD
Fluorescence imaging of (L02, A549, HeLa, and HepG2) cells were recorded by CLFM, respectively. The 6 × 104 cells per well was seeded on a 6-well plate at 37 °C for 24 h. Then, the CMC–FA–CQD nanospheres dispersed in water (0.2 mg mL−1) were added into the cell dishes. After 10 min and 24 h incubation, the nanospheres-loaded cells were repeatedly washed with PBS (10 mM, pH 7.4) to remove free nanospheres absorbed or/and attached on the outer surface of cell membrane. After that, these targeted cells were detected on CLFM for fluorescence imaging (excited at 440 nm).3 Results and discussion
3.1 Synthesis and characterization of CMC–FA and CMC–FA–RBS (CQD)
In this article, we employed a simple carboxy-amine reaction to prepare CMC-graft-FA conjugates (i.e. CMC–FA nanospheres).28 CMC–FA nanospheres dispersed in aqueous solution have positively charged –NH3+ and negatively charged –COO− groups, which indicates a possibility to generate CMC–FA–RBS (RBS is positively charged anion, Fe4S3(NO)7−) and CMC–FA–CQD (CQD is stabilized by cation polyelectrolyte, PEI) hybrid nanospheres (Scheme 1) under electrostatic adsorption interactions.29 The CMC–FA was characterized by 1H-NMR spectroscopy, as exhibited in Fig. 1. The spectrum (Fig. 1a) contains apparent signals deriving from both CMC and FA (Fig. 1b). Fig. 1b displays particular signals of FA. The integral signal ratio at 8.7 ppm corresponds to the proton at 7-position of pterin ring. The signals at 8.2, 7.6, 7.0, 6.6, 4.4, 4.3, 2.5, and 2.0 ppm corresponds to the protons of FA originating from the positions of 18, 13/15, 10, 12/16, 9, 19, 22, and 21, respectively. The signal at 2.3 ppm corresponds to the peak of solvents (HOD/DMSO-d6). In Fig. 1a, the chemical modification (graft) of CMC by FA resulted in the appearance of FA characteristic peaks located at 7.2 ppm (H13/15), 6.3 ppm (H12/16), 2.2 ppm (H22), and 2.0 ppm (H21). In the region from 4.0 to 5.0 ppm, the peaks are overlapped with the peaks of H3–H6 in CMC backbone. Marked increase in the integral intensities of 3.0 ppm (H2′, CMC) and 3.2 ppm (H5′, CMC) were observed, which suggested the FA attaching to CMC actually. The degree of substitution (DS, %) (in the CMC grafted by FA) was determined from the relative peak area ratio of C12/16 of FA (at 6.3 ppm) and C5′ of CMC (at 3.2 ppm). As a result, the maximum value of DS was calculated to be 12.5%.FTIR spectra of CMC and CMC–FA were shown in Fig. 1c. These spectra indicated the successful conjugation between CMC and FA. There are three characteristic peaks of CMC at 3435 cm−1 of ν(OH), 1095 cm−1 of δ(C–O–C) and 1605 cm−1 of ν(NH2).10 CMC–FA spectrum shows the disappearance of NH2-associated band (at 1605 cm−1) of N–H bending in the amine (I), and the occurrence of NH-associated band (at 1575 cm−1) of N–H bending in the amine (II). A new band at 1318 cm−1 was watched, which was ascribed to ν(C–N) in the amine (II). It was inferred that the NH2 groups in CMC could be partly converted into NH groups. In addition, the characteristic peaks located at 1512 and 1379 cm−1 were respectively attributed to the NH3+ groups obtained from FA and residual NH2 groups of CMC, and the –COO− groups obtained from CMC and residual –COOH groups of FA.
The morphology and structure of products were examined by TEM and SEM images, as shown in Fig. 2. The TEM images of CQD (Fig. 2a) showed that the as-prepared PEI-stabilized CQD were uniform, mono-dispersedly spherical particles with an average diameter of 2.5 nm. After electrostatic interactions, CMC–FA–CQD nanospheres were prepared, and exhibited a uniform, discrete spherical shape with the whole approximate diameter of about 120 nm (Fig. 2b). Multiple dark spots (several or tens of particles) were clearly observed in the inner of each particle, which was ascribed to the existence of CQD based on the formation mechanism of CMC–FA–CQD. By contrast, Fig. 2c (for CMC–FA–RBS) did not exhibit the multiple dark spots in each particle because the absence of CQD. The whole gray and spherical particles were watched with an average diameter of about 115 nm. The RBS belongs to inorganic salt anion and cannot be found by TEM and SEM images of nanospheres.26,27 Hence, TEM images of CMC–FA–RBS hybrid nanospheres should be in good agreement with CMC–FA nanospheres. The SEM images of CMC–FA nanospheres (Fig. 2d) showed uniform, monodisperse and spherical particles, and an average diameter of about 110 nm, which is close to that of CMC–FA–RBS, as can be seen in Fig. 2c. Moreover, the average diameter of CMC–FA–CQD measured by dynamic light scattering (DLS) was 175 nm at room temperature, and there was a relatively low diameter polydispersity index (DPI) of 0.06.30,31 As well established, the hydrodynamic diameter of nanospheres from DLS is larger than that from TEM because of hydrate layers in aqueous environment,32 further revealing the appearance of an outer layer of polymers in nanospheres. In terms of the synthesis procedures, the polymers correspond to CMC–FA, and the CQD particles were embedded into CMC–FA networks via electrostatic interactions, thus resulting in the formation of CMC–FA–CQD hybrid nanospheres.
 | ||
| Fig. 2 Wide-field TEM images of (a) PEI-stabilized CQD, (b) CMC–FA–CQD hybrid nanospheres and (c) CMC–FA–RBS hybrid nanospheres, and (d) SEM images of CMC–FA nanospheres. | ||
UV-vis and PL spectra of aqueous suspension of CMC–FA–CQD hybrid nanospheres were characterized. As can be seen in Fig. 3a, no dramatic change in UV-vis and PL spectra of CQD can be observed after encapsulated by CMC–FA polymers, revealing that the CMC–FA can be considered as stabilizers to protect CQD. UV-vis spectrum of CMC–FA–CQD displays a shoulder peak around 440 nm, while the corresponding PL spectrum exhibits a sharp peak located at 510 nm (inserted in Fig. 3a, bright PL emission excited at 365 nm). The two peak positions are consistent with the single CQD without the encapsulation of CMC–FA. Furthermore, CMC–FA–CQD nanospheres showed well-stabilized dispersion in PBS, and the corresponding PL intensity could be maintained even for several weeks (Fig. S1 in ESI†), indicating a significant potential in PL imaging application of targeted tumor cells. Fig. 3b illustrates UV-vis spectrum of CMC–FA–RBS nanospheres, showing a clear shoulder peak around 375 nm. The peak position is the characteristic peak of RBS in aqueous media,27 which suggested that the embedding of RBS into CMC–FA networks caused neglectful change in characteristic absorption spectrum of RBS. As inserted in Fig. 3b, the RBS (referring to its chemical structure) can cause photolysis under light irradiation of UV-vis wavelengths, thus resulting in the release of NO at biological relevant concentrations.33,34 The inserted equation defines the stoichiometry and mechanisms of photoreaction pathway in aerobic, aqueous media.35,36 Nevertheless, aqueous RBS is stable in the dark. Provide that the CMC–FA–RBS nanospheres are excited by a laser (e.g. 365 nm), the light excitation will immediately trigger NO release. In other words, the CMC–FA–RBS nanospheres could be developed toward an effective nanocarrier for controlled release of NO.
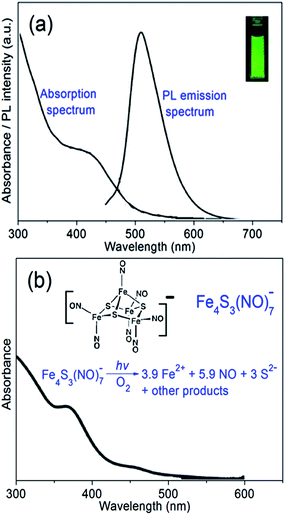 | ||
| Fig. 3 (a) UV-vis absorption and PL emission (excited at 440 nm) spectra of CMC–FA–CQD, and (b) UV-vis absorption spectrum of CMC–FA–RBS. The inset in (a) is fluorescence image (excited at 365 nm) of CMC–FA–CQD (1 mg mL−1) in PBS (10 mM, pH 7.4). The inset in (b) is stoichiometry and mechanism of the photoreaction pathway of RBS in aerobic, aqueous media.34,35 | ||
3.2 Colloidal stability and cytotoxicity of CMC–FA–RBS and CMC–FA–CQD
Colloidal stabilities of the prepared CMC–FA–RBS and CMC–FA–CQD hybrid nanospheres were evaluated through incubating them in PBS and BSA at room temperature (∼20 °C). As shown in Fig. 4a, less than 9% of decrease in absorbance of aqueous suspension of CMC–FA–RBS was observed after incubating with 10 mM of PBS (from pH 4.9 to 9.2) for 10 min in the dark. Under the identical conditions as the case of CMC–FA–RBS, no more than 8% of increase in PL intensity of aqueous suspension of CMC–FA–CQD was observed. Although more than 90% of the initial absorbance and PL intensity were preserved (Fig. 4a), 10 min of incubation is not enough to confirm the stabilities of products. Hence, related experiments referring to longer incubation were regularly conducted. After 48 h incubation, Fig. 4b demonstrated only less than 12% of fluctuation (decrease in absorbance and increase in PL intensity) for the two samples. These results suggested that CMC–FA–RBS and CMC–FA–CQD hybrid nanospheres dispersed in water were highly stable, exhibiting a potential for biological and biomedical applications.Commonly, cytotoxicity of the newly prepared nanomaterials should be first investigated before their biological applications. According to this principle, the cytotoxicity of CMC–FA–RBS and CMC–FA–CQD nanospheres was studied using the standard protocols of cell viability.31 Herein, human hepatocytes L02 (normal) and HeLa (tumor) cell lines were chosen to incubate with CMC–FA–RBS and CMC–FA–CQD in vitro. Experimental results showed relatively high cell viability, as exhibited in Fig. 4c and d. After 24 h and 48 h incubation with CMC–FA–RBS and CMC–FA–CQD in the concentration range from 0 to 1 mg mL−1 at the physiological temperature (37 °C), the values of L02 and HeLa cell viability were consistent with the controls except for less than 5% of fluctuation. The results revealed a low cytotoxicity and favorable cell compatibility for CMC–FA–RBS and CMC–FA–CQD hybrid nanospheres even at a concentration up to 1 mg mL−1. Additionally, the viability of HeLa cells was slightly lower (∼2%) than that of L02 cells under the same conditions, which indicated that these nanospheres possessed a higher growth inhibition for tumor cells than normal cells due to the FR-mediated specific endocytosis.2,12
3.3 Light-triggered NO release from CMC–FA–RBS
To demonstrate the feasibility of light-triggered NO release, the CMC–FA–RBS nanospheres were dispersed in PBS (10 mM, pH 7.4) at ambient temperature with vigorous stirring to generate aqueous suspension, which was then continuously irradiated with a diode laser (365 nm, 1 W). The aqueous suspension was entrained with He, and the concentrations of released NO in aqueous suspension were measured by using the method of colorimetric Griess reaction (Part S2 in ESI†). As shown in Fig. 5a, light excitation for short time intervals (0–300 s) led to bursts of NO release (more than 70%). Longer excitation (300–600 s) only triggered a little more (∼3%) release of NO, suggesting that the NO release from CMC–FA–RBS is almost complete after 300 s light excitation. Repeated NO release was performed by commutative excitation and storing in the dark at 60 s of time intervals. As inserted in Fig. 5a, the NO release rapidly increases due to light excitation in the first release process, but then it nearly pauses (only less than 2% of increase was watched) after storing in the dark. Similar variations maintain in the subsequent release circles, indicating that the NO release from CMC–FA–RBS is attributed to light excitation that induces the photolysis of RBS in aqueous media.To further testify the light-triggered NO release from CMC–FA–RBS nanospheres, related experiments referring to CMC–FA–RBS in the dark and CMC–FA under light excitation were conducted, and experimental results were used for the control groups, as displayed in Fig. 5b. Under 0–600 s of light excitation (365 nm), no release of NO from CMC–FA nanospheres was detected. CMC–FA–RBS nanospheres in the dark (without light excitation) only released less than 2.5% of NO after 600 s of light excitation. These results revealed that upon light excitation, the blank CMC–FA nanospheres cannot release NO, and the RBS-embedded CMC–FA nanospheres in the dark only release neglectful amount of NO. In view of these results, CMC–FA–RBS nanospheres could be further developed toward a smart “on–off” switch for NO release by visible light (e.g. 365 nm) excitation, and the release amounts and concentrations of NO from the CMC–FA–RBS nanospheres could be controlled by the light excitation time and intensity. The schematic illustration of CMC–FA–RBS hybrid nanosphere system for light-triggered release of NO is available (Fig. S2 in ESI†).
3.4 Targeted fluorescence imaging from CMC–FA–CQD in vitro
To test the feasibility of CMC–FA–CQD hybrid nanospheres for targeted fluorescence imaging of FR-expressed tumor cells, HeLa cells (a kind of human cervical carcinoma cell with a high growth rate and over-expression of FR), HepG2 cells (a human liver carcinoma cell line with a high-expression of FR), A549 cells (adenocarcinomic human alveolar basal epithelial cells for the FR negative control), and L02 cells (a human normal hepatocytes cell without expression of FR),2,37 were selected and incubated with the CMC–FA–CQD nanospheres. After 10 min incubation, both the fluorescent cell amounts and fluorescence intensities for HepG2 cells (Fig. 6c) and HeLa cells (Fig. 6d) were more remarkable than that of L02 cells (Fig. 6a) and A549 cells (Fig. 6b), which suggested that the uptake of nanospheres into HepG2 cells and HeLa cells were more significant than L02 cells and A549 cells. In general, the FA-conjugated nanoparticles are transported into tumor cells by a FR-mediated endocytosis mechanism because of the high expression of FR on the surface of tumor cells.2 The fluorescent cell amounts and brightness of fluorescence imaging was in the order of L02 < A549 < HepG2 < HeLa cells after a short incubation (10 min). The results are consistent with their capacity of FR expression because the L02 cells and A549 cells have no obvious expression of FR, and HepG2 cells have high-expression of FR, and HeLa cells have over-expression of FR.38–40 In addition, corresponding imaging experiments were carried out after a longer incubation (24 h). Results displayed that the imaging capacity of HeLa cell groups was still markedly higher than other cell groups (in Fig. S3, ESI†). In terms of the above results, the CMC–FA–CQD hybrid nanosphere system (its schematic illustration is available in Fig. S4, ESI†) has been proved its efficiency for fluorescence imaging in HeLa cells, exhibiting a potential for targeted fluorescence imaging of tumor cells with FR over-expression.4 Conclusions
In this study, we presented a facile aqueous synthesis of CMC–FA–RBS and CMC–FA–CQD hybrid nanospheres via electrostatic adsorption interactions between CMC–FA grafted conjugates and RBS (CQD). The prepared nanospheres were regularly characterized, and further developed toward light-triggered release of NO and targeted fluorescence imaging in tumor cells. Experimental results confirmed that these nanospheres possessed highly colloidal stabilities in PBS (pH 4.9–9.2) and BSA (0–48 h incubation), and exhibited neglectful cytotoxicity in the concentration range of nanospheres (0–1 mg mL−1). CMC–FA–RBS nanospheres as drug carriers were used for visible light (365 nm) triggered release of NO, showing a smart “on–off” switch for controlled NO release. CMC–FA–CQD nanospheres as effective probes were used for targeted fluorescence imaging in tumor (e.g. HeLa) cells with over-expression of FR. Experimental results from the studies of hybrid nanospheres would facilitate the design and preparation of other multifunctional nanospheres, as well as their potential applications, especially serving as effective nanocarriers for controlled drug release, targeted cell imaging, in vivo diagnosis and therapy of tumors.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (grant no. 51173104), the Nanotechnology Program of Science and Technology Committee of Shanghai (grant no. 11nm0503500), the Postdoctoral Science Foundation of China (grant no. 2013M531164), and the Postdoctoral Science Foundation of Shanghai (grant no. 13R21413800).Notes and references
- S. B. Chen, H. Zhong, L. L. Zhang, Y. F. Wang, Z. P. Cheng, Y. L. Zhu and C. Yao, Carbohydr. Polym., 2010, 82, 747–752 CrossRef CAS PubMed.
- J. M. Shen, W. J. Tang, X. L. Zhang, T. Chen and H. X. Zhang, Carbohydr. Polym., 2012, 88, 239–249 CrossRef CAS PubMed.
- G. Y. Lu, L. J. Kong, B. Y. Sheng, G. Wang, Y. D. Gong and X. F. Zhang, Eur. Polym. J., 2007, 43, 3807–3818 CrossRef CAS PubMed.
- L. C. Wang, X. G. Chen, L. J. Yu and P. W. Li, Polym. Eng. Sci., 2007, 47, 1373–1379 CAS.
- X. T. Xue and J. X. He, Carbohydr. Polym., 2009, 75, 203–207 CrossRef CAS PubMed.
- L. Tan, A. Wan, H. Li and Q. Lu, Acta Biomater., 2012, 8, 3744–3753 CrossRef CAS PubMed.
- S. Okarvi and I. Jammaz, Cancer Biother. Radiopharm., 2006, 21, 49–60 CrossRef CAS PubMed.
- F. Liu, D. Deng, X. Chen, Z. Qian, S. Achilefu and Y. Gu, Mol. Imag. Biol., 2010, 12, 595–607 CrossRef PubMed.
- M. D'Angelica, J. Ammori, M. Gonen, D. S. Klimstra, P. S. Low, L. Murphy, M. R. Weiser, P. B. Paty, Y. Fong, R. P. DeMatteo, P. Allen, W. R. Jarnagin and J. Shia, Mod. Pathol., 2011, 24, 1221–1228 CrossRef PubMed.
- A. Wan, Y. Sun and H. Li, Int. J. Biol. Macromol., 2008, 43, 415–421 CrossRef CAS PubMed.
- S. Wang, J. Luo, D. A. Lantrip, D. J. Waters, C. J. Mathias, M. A. Green, P. L. Fuchs and P. S. Low, Bioconjugate Chem., 1997, 8, 673–679 CrossRef CAS PubMed.
- M. E. Mathew, J. C. Mohan, K. Manzoor, S. V. Nair, H. Tamura and R. Jayakumar, Carbohydr. Polym., 2010, 80, 442–448 CrossRef CAS PubMed.
- D. Bhattacharya, M. Das, D. Mishra, I. Banerjee, S. K. Sahu, T. K. Maiti and P. Pramanik, Nanoscale, 2011, 3, 1653–1662 RSC.
- Y. Zhang, G. Hong, Y. Zhang, G. Chen, F. Li, H. Dai and Q. Wang, ACS Nano, 2012, 6, 3695–3702 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455–458 CrossRef CAS PubMed.
- S. T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y. P. Sun, J. Am. Chem. Soc., 2009, 131, 11308–11309 CrossRef CAS PubMed.
- D. Pan, L. Guo, J. Zhang, C. Xi, Q. Xue, H. Huang, J. Li, Z. Zhang, W. Yu, Z. Chen, Z. Li and M. Wu, J. Mater. Chem., 2012, 22, 3314–3318 RSC.
- S. Moncada and A. Higgs, N. Engl. J. Med., 1993, 30, 2002–2012 Search PubMed.
- M. A. Marletta, M. A. Tayeh and J. M. Hevel, BioFactors, 1990, 2, 219–225 CAS.
- F. C. Fang, J. Clin. Invest., 1997, 99, 2818–2825 CrossRef CAS PubMed.
- J. A. Hrabie and L. K. Keefer, Chem. Rev., 2002, 102, 1135–1154 CrossRef CAS PubMed.
- P. G. Wang, M. Xian, X. Tang, X. Wu, Z. Wen, T. Cai and A. J. Janczuk, Chem. Rev., 2002, 102, 1091–1134 CrossRef CAS PubMed.
- S. Griveau and F. Bedioui, Analyst, 2013, 138, 5173–5181 RSC.
- D. Seyferth, M. K. Gallagher and M. Cowie, Organometallics, 1986, 5, 539–548 CrossRef CAS.
- M. Lewin, K. Fisher and I. Dance, Chem. Commun., 2000, 947–948 RSC.
- J. V. Garcia, J. Yang, D. Shen, C. Yao, X. Li, R. Wang, G. D. Stucky, D. Zhao, P. C. Ford and F. Zhang, Small, 2012, 8, 3800–3805 CrossRef CAS PubMed.
- R. Gui, A. Wan, H. Jin, H. Li and C. Zhou, Mater. Lett., 2013, 96, 20–23 CrossRef CAS PubMed.
- R. Gui, X. An and W. Huang, Anal. Chim. Acta, 2013, 767, 134–140 CrossRef CAS PubMed.
- B. Chu, Z. L. Wang and J. Q. Yu, Macromolecules, 1991, 24, 6832–6838 CrossRef CAS.
- R. Gui, Y. Wang and J. Sun, Colloids Surf., B, 2014, 113, 1–9 CrossRef CAS PubMed.
- C. Liu, J. Guo, W. Wang, J. Hu, C. Wang and S. Hu, J. Mater. Chem., 2009, 19, 4764–4770 RSC.
- F. W. Flitney, I. L. Megson, D. E. Flitney and A. R. Butler, Br. J. Pharmacol., 1992, 107, 842–848 CrossRef CAS PubMed.
- F. W. Flitney, I. L. Megson, J. L. M. Thomson, G. D. Kennovin and A. R. Butler, Br. J. Pharmacol., 1996, 117, 1549–1557 CrossRef CAS PubMed.
- J. Bourassa, W. DeGraff, S. Kudo, D. A. Wink, J. B. Mitchell and P. C. Ford, J. Am. Chem. Soc., 1997, 119, 2853–2860 CrossRef CAS.
- J. Bourassa, B. Lee, S. Bernard, J. Schoonover and P. C. Ford, Inorg. Chem., 1999, 38, 2947–2952 CrossRef CAS PubMed.
- Y. Zhang, J. M. Liu and X. P. Yan, Anal. Chem., 2013, 85, 228–234 CrossRef CAS PubMed.
- B. Xue, D. W. Deng, J. Cao, F. Liu, X. Li, W. Akers, S. Achilefu and Y. Q. Gu, Dalton Trans., 2012, 41, 4935–4947 RSC.
- N. Singh, S. Charan, K. Sanjiv, S. H. Huang, Y. C. Hsiao, C. W. Kuo, F. C. Chien, T. C. Lee and P. L. Chen, Bioconjugate Chem., 2012, 23, 421–430 CrossRef CAS PubMed.
- F. Liu, D. W. Deng, X. Y. Chen, Z. Y. Qian, S. Achilefu and Y. Q. Gu, Mol. Imag. Biol., 2010, 12, 595–607 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of PEI-stabilized CQD, determination method of NO concentration, PL stability of CMC–FA–CQD nanospheres, schematic illustration of light-triggered NO release and targeted fluorescence imaging in tumor cells. See DOI: 10.1039/c4ra03034f |
| This journal is © The Royal Society of Chemistry 2014 |