Copper-templated synthesis of gold microcages for sensitive surface-enhanced Raman scattering activity†
Chuncai Kong,
Jian Lv,
Shaodong Sun,
Xiaoping Song and
Zhimao Yang*
School of Science, Key Laboratory of Shannxi for Advanced Materials and Mesoscopic Physics, State Key Laboratory for Mechanical Behavior of Materials, Xi'an Jiaotong University, Collaborative Innovation Center of Suzhou Nano Science and Technology, Xi'an 710049, ShaanXi, People's Republic of China. E-mail: zmyang@mail.xjtu.edu.cn
First published on 3rd June 2014
Abstract
We have demonstrated a facile copper-templated approach for synthesis of nanoparticle-aggregated hollow gold microcages. The as-prepared gold microcages, as active substrates, exhibit remarkable surface-enhanced Raman scattering activity for 4-mercaptobenzoic acid.
Surface-enhanced Raman spectroscopy (SERS) has been attracting significant attention since its discovery in the 1970s.1 It is a powerful technique in chemical and biological analyses at the single-molecule level because of its stability, sensitivity and anti-interference properties.2 To date, numerous assemblies of noble metals have been synthesized as active substrates for SERS, including Au flowers,3 Au nanopatches,4 porous Ag,5 Au–Ag nanocages,6 Au@Pd nanocrystals,7 aggregates of Au nanoparticles8 and polymer – Au hybrids.9 Both experimental measurements and theoretical simulations have shown that the SERS enhancement depended mostly on the substrate features, including the size, shape and composition.3,10 “Hot spots”, which are localized regions of enhanced electromagnetic fields, are essential for the substrate to be SERS sensitive. On the surface of nanoparticle-aggregated hollow structures, there are usually many nanogaps and protuberances, which can provide a high density of such hot spots3,5,11 and lead to high performance using SERS.
Hollow Au micro- and nanostructures have been widely applied in sensors,12 catalysis,13 and biomedical therapy,14 because of their chemical stability and good biocompatibility. Compared with solid structures, the hollow ones are attractive because of their high specific surface areas, low densities, hollow interiors and low cost.15 Furthermore, their surface morphologies are crucial for SERS performance. Thus, the design and synthesis of hollow Au architectures with a lower cost and facile synthetic method is still needed.
Galvanic replacement has been widely used as a simple and effective method to produce nanomaterials with various mophologies. The structure and morphology of the final hollow structures can be accurately controlled in the galvanic displacement reaction by manipulating the reaction conditions and using micro/nanoparticles as sacrificial templates. Our group has prepared cauliflower-like and paddy-like copper nanostructures using a zinc foil as a reductant.16 Yang et al. reported the synthesis of noble metal alloy mesocages with Cu2O as the sacrificial templates.17 Xia et al. obtained Au nanocages by using Ag nanocubes as the templates.18 But there are few reports about the synthesis of Au hollow structures with Cu as the sacrificial template. Lu et al. obtained Au nanotubes by using chloroauric acid (HAuCl4) with Cu nanowires.19 And in another example, Xie et al. reported that AuxCu1−x nanocages and nanotubes could be formed by galvanic replacement between the Cu nanostructures and HAuCl4.20
A difference of electric potential drives the galvanic replacement reaction. Because the standard reduction potential Eθ of (Cu2+/Cu), which is equal to +0.342 V (vs. a standard hydrogen electrode), is lower than the Eθ of (AuCl4−/Au), which is equal to +0.99 V,18b Au can be easily reduced by the Cu templates. In this paper, we present the synthesis of Au microcages using the galvanic replacement reaction between hollow Cu microcages and an HAuCl4 aqueous solution. The as-prepared Au microcages displayed good SERS activity for 4-mercaptobenzoic acid (4-MBA) because of the protuberances and nanogaps between the neighboring nanoparticles.
Our strategy to achieve the hollow Au microcages is based on a reduction/etching route. They can be synthesized by a two-step process: the formation of hollow Cu architectures via a Cu2O-templated solution route (see Fig. S1†), followed by the sacrifice of the hollow Cu templates by a facile galvanic replacement reaction (see Scheme 1). In a typical synthesis, carried out in a conical flask, 3.84 mg of the as-prepared hollow Cu microstructures were dispersed in 15 mL of deionized water, which contained 0.05 g polyvinylpyrrolidone. 10 min later, 2 ml of an aqueous HAuCl4 solution (20 mM) were added dropwise. Finally, a wine colored solution was obtained after running the reaction for 30 min at room temperature. Then the product was cleaned with deionized water and ethanol by repeated centrifugation. The final product was dried at 50 °C in a vacuum drying oven for further characterization and measurement.
 | ||
| Scheme 1 A schematic illustration of the synthesis of hollow Au microcages using hollow Cu templates. | ||
SERS of the as-prepared products were acquired using an HR800 Raman spectrometer (HORIBA Jobin Yvon) with a charge-coupled device (CCD) detector and He–Ne laser (633 nm). The SERS spectra were collected using a 100× objective and an accumulation time of 20 s. In addition, the grating was 600 g mm−1 and the filter in the SERS spectra was D1. The as-prepared hollow Au microcages were added into 1 mL of 4-MBA ethanol solution at different concentrations. The solutions were mixed ultrasonically for 3 min and left undisturbed for another 2 h. A portion (20 μL) of the solution was transferred onto a glass substrate and then dried for the SERS measurements.
Hollow Au microcages were obtained by the galvanic replacement reaction approach described above using HAuCl4 as the Au source and hollow Cu microcages as the reductant. Fig. S2a† shows the X-ray diffraction (XRD) pattern of the as-prepared product, and the sharp peaks at 38.3°, 44.5°, 64.7° and 77.7° are indexed to the diffraction from the (111), (200), (220) and (311) planes of face-centered cubic (fcc) Au (JCPDS file no. 65-8601). No peaks for impurities were detected, suggesting that as-obtained Au microcages were of high purity. The purity and the composition of the as-prepared Au microcages were further investigated by X-ray photoelectron spectroscopy (XPS). As shown in Fig. S2b,† the peaks located at 83.6 eV and 87.2 eV were assigned to the core-levels Au 4f7/2 and Au 4f5/2, respectively, corresponding to those of Au0.21 The morphology and structure were investigated by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). As shown in Fig. 1a, the Au microcages are well monodispersed and have an average size of 2.3 ± 0.3 μm (Fig. S3a†). The inset of Fig. 1a displays the scanning electron microscopy (SEM) image of a broken Au microcage particle, which suggests that the product has a hollow interior. Fig. 1b and c show that the Au microcage consists of numerous small particles with an average size of 42.2 ± 7.5 nm (Fig. S3b†). The raised particles on the surface would enhance the SERS activity. The hollow interior and rough surface were further observed by TEM images (as shown in Fig. 1d and e). The lattice spacing shown in the HRTEM image (Fig. 1f) is 0.236 nm which agrees well with the spacing between the (111) planes of Au. Fig. S4† shows the UV-vis spectra of Au microcages in the presence and absence of added 4-MBA. The spectra are dominated by the surface plasmon resonance of the Au microcages, and there is no observable change after addition of 4-MBA.
4-MBA was selected as the probe molecule to study the SERS activity of as-prepared Au microcages. SERS spectra of 4-MBA at different concentrations adsorbed on to Au microcages are shown in Fig. 2, and they are similar to those obtained in previous research.11,22 The intrinsic peaks observed at about 1078 cm−1 and 1589 cm−1 are attributed to the ν8a and ν12 aromatic ring vibrations, respectively.11,23,24 There are some differences between the Raman shifts of solid 4-MBA and 4-MBA on Au microcages in the SERS spectra, suggesting a strong interaction of 4-MBA with the Au surface.25 Fig. S5† shows the linear relationship between the intensities at 1589 cm−1 and the logarithmic concentration of 4-MBA. The Raman intensities vary linearly with the concentration of 4-MBA in the range of 10−7 to 10−4 M with a correlation factor of 0.985. This linear plot provides a calibration graph for the quantitative detection of 4-MBA.
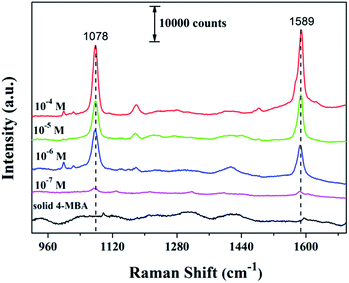 | ||
| Fig. 2 Raman spectrum of solid 4-MBA and the SERS spectra of 4-MBA with different concentrations adsorbed on Au microcages. | ||
To further evaluate the enhancement ability of the Au microcages, the SERS enhancement factor (EF) was estimated based on the following equation:4,23
| EF = (ISERS/NSERS)/(Ibulk/Nbulk) |
In this equation, ISERS and Ibulk are the Raman scattering intensities of 4-MBA on the surface of Au microcages and of the normal spectrum of bulk 4-MBA, respectively. NSERS and Nbulk are the number of 4-MBA molecules under the detection spot (1 × 1 μm2) for the Au sample and bulk sample, respectively. The measured Raman scattering intensities for 10−4 M, 10−5 M, 10−6 M and bulk 4-MBA at 1589 cm−1 are 25222, 15342, 11858 and 1183, respectively. According to the penetration depth of the focused laser (13 μm) and the bulk density of 4-MBA (1.5 g cm−3), Nbulk = 1.13 × 1011. The number density of the nanoparticles is about 410 μm−2. For 10−4 M 4-MBA, the packing density adsorbed on the surface is ∼3.01 × 105 molecules per μm−2.21,26 On this basis, the value of NSERS = 3.4 × 105, and the EF was then calculated to be 7.1 × 106 based on the peak at 1589 cm−1. By the same method, the EF values are estimated to be 4.31 × 106 and 3.33 × 106 for 10−5 M 4-MBA and 10−6 M 4-MBA, respectively. The EF values obtained are comparable to those values obtained for other Au nanostructures.27
Conclusions
In summary, we have successfully synthesized nanoparticle-aggregated Au microcages by using hollow Cu microstructures as the sacrificial templates. The SERS activity of Au microcages as substrates were further evaluated by using 4-MBA as the probe molecule. The values of EF were about 106, indicating that the Au microcage is a promising SERS substrate for sensor detection.Acknowledgements
This work was supported by National Science Foundation of China (NSFC no. 51272209 and 51302213), Doctoral Fund of Ministry of Education of China (no. 20120201120051), Program for Key Science and Technology Innovative Team of Shaanxi Province (no. 2013KCT-05), Youth Foundation of Shaanxi Province of China (no. 2012JQ6007), and Fundamental Research Funds for the Central Universities of China.Notes and references
- (a) M. Fleischmann, P. J. Hendra and A. J. McQuillan, Chem. Phys. Lett., 1974, 26, 163–166 CrossRef CAS; (b) D. L. Jeanmaire and R. P. Van Duyne, J. Electroanal. Chem. Interfacial Electrochem., 1977, 84, 1–20 CrossRef CAS.
- M. D. Porter, R. J. Lipert, L. M. Siperko, G. F. Wang and R. Narayanan, Chem. Soc. Rev., 2008, 37, 1001–1011 RSC.
- M. Pradhan, J. Chowdhury, S. Sarkar, A. K. Sinha and T. Pal, J. Phys. Chem. C, 2012, 116, 24301–24313 CAS.
- J. He, P. Zhang, J. L. Gong and Z. H. Nie, Chem. Commun., 2012, 48, 7344–7346 RSC.
- X. Luo, S. M. Lian, L. Q. Wang, S. C. Yang, Z. M. Yang, B. J. Ding and X. P. Song, CrystEngComm, 2013, 15, 2588–2591 RSC.
- M. Rycenga, K. K. Hou, C. M. Cobley, A. G. Schwartz, P. H. C. Camargo and Y. N. Xia, Phys. Chem. Chem. Phys., 2009, 11, 5903–5908 RSC.
- L. F. Zhang and C. Y. Zhang, Nanoscale, 2013, 5, 6074–6080 RSC.
- I. Blakey, Z. Merican and K. J. Thurecht, Langmuir, 2013, 29, 8266–8274 CrossRef CAS PubMed.
- (a) P. Dey, I. Blakey, K. J. Thurecht and P. M. Fredericks, Langmuir, 2014, 30, 2249–2258 CrossRef CAS PubMed; (b) P. Dey, I. Blakey, K. J. Thurecht and P. M. Fredericks, Langmuir, 2012, 29, 525–533 CrossRef PubMed.
- M. A. Mahmoud, Langmuir, 2013, 29, 6253–6261 CrossRef CAS PubMed.
- S. W. Li, P. Xu, Z. Q. Ren, B. Zhang, Y. C. Du, X. J. Han, N. H. Mack and H. L. Wang, ACS Appl. Mater. Interfaces, 2012, 5, 49–54 Search PubMed.
- M. A. Mahmoud and M. A. El-Sayed, J. Am. Chem. Soc., 2010, 132, 12704–12710 CrossRef CAS PubMed.
- X. W. Liu, RSC Adv., 2011, 1, 1119–1125 RSC.
-
(a) J. B. Song, J. J. Zhou and H. W. Duan, J. Am. Chem. Soc., 2012, 134, 13458–13469 CrossRef CAS PubMed;
(b) S.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E Skrabalak, J. Y. Chen, L. Au, X. M. Lu, X. D. Li and Y. N. Xia, Adv. Mater., 2007, 19, 3177–3184 CrossRef PubMed.
E Skrabalak, J. Y. Chen, L. Au, X. M. Lu, X. D. Li and Y. N. Xia, Adv. Mater., 2007, 19, 3177–3184 CrossRef PubMed. - X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- (a) C. C. Kong, S. D. Sun, J. Zhang, H. D. Zhao, X. P. Song and Z. M. Yang, CrystEngComm, 2012, 14, 5737–5740 RSC; (b) S. D. Sun, C. C. Kong, L. Q. Wang, S. C. Yang, X. P. Song, B. J. Ding and Z. M. Yang, CrystEngComm, 2011, 13, 1916–1921 RSC.
- F. Hong, S. D. Sun, H. J. You, S. C. Yang, J. X. Fang, S. W. Guo, Z. M. Yang, B. J. Ding and X. P. Song, Cryst. Growth Des., 2011, 11, 3694–3697 CAS.
- (a) S. E. Skrabalak, J. Y. Chen, Y. G. Sun, X. M. Lu, L. Au, C. M. Cobley and Y. N. Xia, Acc. Chem. Res., 2008, 41, 1587–1595 CrossRef CAS PubMed; (b) L. Au, X. M. Lu and Y. N. Xia, Adv. Mater., 2008, 20, 2517–2522 CrossRef CAS PubMed.
- X. F. Lu, M. McKiernan, Z. M. Peng, E. P. Lee, H. Yang and Y. N. Xia, Sci. Adv. Mater., 2010, 2, 413–420 CrossRef CAS PubMed.
- S. F. Xie, M. S. Jin, J. Tao, Y. C. Wang, Z. X. Xie, Y. M. Zhu and Y. N. Xia, Chem.–Eur. J., 2012, 18, 14974–14980 CrossRef CAS PubMed.
- (a) Z. Y. g. Huo, C. K. Tsung, W. Y. Huang, X. F. Zhang and P. D. Yang, Nano Lett., 2008, 8, 2041–2044 CrossRef CAS PubMed; (b) Z. G. Xiong, L. L. Zhang, J. Z. Ma and X. S. Zhao, Chem. Commun., 2010, 46, 6099–6101 RSC; (c) J. B. Zhao, H. Liu, S. Ye, Y. M. Cui, N. H. Xue, L. M. Peng, X. F. Guo and W. P. Ding, Nanoscale, 2013, 5, 9546–9552 RSC.
- P. N. Sisco and C. J. Murphy, J. Phys. Chem. A, 2009, 113, 3973–3978 CrossRef CAS PubMed.
- L. Jiang, T. T. You, P. G. Yin, Y. Shang, D. F. Zhang, L. Guo and S. H. Yang, Nanoscale, 2013, 5, 2784–2789 RSC.
- Y. S. Li, H. M. Su, K. S. Wong and X. Y. Li, J. Phys. Chem. C, 2010, 114, 10463–10477 CAS.
- (a) M. I. Dar, S. Sampath and S. A. Shivashankar, J. Mater. Chem., 2012, 22, 22418–22423 RSC; (b) C. C. Kong, S. D. Sun, X. Z. Zhang, X. P. Song and Z. M. Yang, CrystEngComm, 2013, 15, 6136–6139 RSC; (c) J. C. Santos Costa, R. A. Ando, A. C. Sant'Ana, L. M. Rossi, P. S. Santos, M. L. A. Temperini and P. Corio, Phys. Chem. Chem. Phys., 2009, 11, 7491–7498 RSC.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Anal. Chem., 2005, 77, 3261–3266 CrossRef CAS PubMed.
- L. Osinkina, T. Lohmüller, F. Jäckel and J. Feldmann, J. Phys. Chem. C, 2013, 117, 22198–22202 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern and FESEM images of Cu2O and the hollow Cu, XRD pattern, XPS spectrum, size distribution and UV-vis spectra of the as-prepared Au microcages. The linear relationship between the intensities at 1589 cm−1 and the logarithmic concentration of 4-MBA. See DOI: 10.1039/c4ra03027c |
| This journal is © The Royal Society of Chemistry 2014 |

