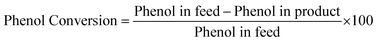DOI:
10.1039/C4RA03004D
(Paper)
RSC Adv., 2014,
4, 32467-32474
Selective synthesis of propofol (2,6-diisopropylphenol), an intravenous anesthetic drug, by isopropylation of phenol over H-beta and H-mordenite
Received
4th April 2014
, Accepted 27th June 2014
First published on 30th June 2014
Abstract
Propofol (2,6-diisopropylphenol/DIPP) is the world's most widely used intravenous general anesthetic and is typically synthesized by isopropylation of phenol over an acid catalyst. It is highly difficult to stabilize bio-oil containing phenolic compounds. The isopropylation of this phenol (a model compound representing species in bio-oils) is one of the options to stabilize the bio-oil and convert it into valuable products. Probably for the first time, H-beta- and H-mordenite-catalysed vapour phase isopropylation of phenol with isopropyl alcohol (IPA) was studied to selectively synthesize DIPP. The optimization of various operating parameters such as molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream were performed. H-beta (94% phenol conv. and 56% DIPP sel.) was found to be a potential and more active catalyst than H-mordenite (68% phenol conv. and 43% DIPP sel.) at optimized process parameters. A kinetic model is proposed to probe the intricate reaction kinetics and validated (R2 > 0.98) by the experimental results. H-beta catalyst was observed to be stable for more than 25 h with 94% phenol conversion and 56% selectivity towards DIPP at optimized process parameters. The phenol conversion and DIPP selectivity obtained in the present study are higher than those reported so far. The activation energy obtained for isopropylation of phenol with IPA over H-beta is calculated to be 25.39 kJ mol−1.
IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream were performed. H-beta (94% phenol conv. and 56% DIPP sel.) was found to be a potential and more active catalyst than H-mordenite (68% phenol conv. and 43% DIPP sel.) at optimized process parameters. A kinetic model is proposed to probe the intricate reaction kinetics and validated (R2 > 0.98) by the experimental results. H-beta catalyst was observed to be stable for more than 25 h with 94% phenol conversion and 56% selectivity towards DIPP at optimized process parameters. The phenol conversion and DIPP selectivity obtained in the present study are higher than those reported so far. The activation energy obtained for isopropylation of phenol with IPA over H-beta is calculated to be 25.39 kJ mol−1.
1 Introduction
Recently, processing of renewable raw materials from ligno-cellulosic biomass into value added chemicals and fuels has gained much attention from researchers.1–3 The bio-oils obtained by fast pyrolysis of lignin are highly viscous, very corrosive and have low heating value; hence, they require upgrading (further processing) to be applicable as fuel.4 In order to improve these qualities, hydro-deoxygenation processes, which increase the H/C molar ratio and decrease the O/C molar ratio, have been utilized.5 However, due to difficulties in complete removal of oxygen, the phenolic compounds often exist in the final bio-oil composition. An alkylation has been used to further improve bio-oils by enhancing their H/C ratios.3,6 Alkylated phenols obtained from bio-oil could serve as useful chemicals for industrial applications.
In addition, isopropylation of this phenol (a model compound representing species in bio-oils) would be a green process to produce industrially important isopropylphenol (IPP). IPP finds extensive applications in flavouring agents, adhesives, agricultural chemicals and pharmaceuticals.3,7–15 In particular, isopropylation of phenol with isopropyl alcohol (IPA) also produces 2,6-diisopropylphenol (DIPP/propofol), a very important drug.9–12 Propofol (DIPP) is the world's most widely used intravenous general anaesthetic;16 moreover, it is the active ingredient in Diprivan. Furthermore, the isopropyl group on the ring can be reacted to make phenol carboxylic acids and esters, opening up new avenues for many synthetic routes. In this work, we have evaluated the isopropylation of phenol, a model compound representing species in bio-oils with IPA catalysed by H-beta and H-mordenite zeolites, aiming to selective synthesize propofol.
To the best of our knowledge, open literature on the isopropylation of phenol to propofol is very limited.3,7–15 Wei et al.13 reported 61% phenol conversion and 70% IPP selectivity over MCM-49 at optimized reaction conditions. Wang et al.14 reported the use of a hierarchically structured ZSM-5 zeolite of c-axis-oriented nano-rods in a continuous alkylation of phenol with isopropanol. They obtained a maximum phenol conversion of 58% and a maximum yield of 40% IPP over NZ160-08 catalyst.13 Recently, Wang et al.15 used modified SAPO-11 zeolites as catalysts for the alkylation of phenol with IPA and reported 55% phenol conversion and 77% selectivity towards IPP. All these reports led to the formation of IPP not DIPP.13–15 There are very few reports on the synthesis of DIPP.3,7–12 Xu et al.3 have used various types of zeolites such as Y, BEA, MOR, ZSM-5, TNU-9 and ZSM-11 for gas phase alkylation of phenol with propylene. Alkylation of phenol with propylene has been reported over H-β, USY and Cs+ exchanged HZSM.7,8 Vapour phase alkylation of phenol with isopropyl acetate has been performed over Zn–Al-MCM-41, Al-MCM-41 and Fe–Al-MCM-41 catalysts.9,10 Isopropylation of phenol with IPA has been reported over 20% (w/w) Cs2.5H0.5PW12O40/K-10 (ref. 11) and Al3O3 catalyst.12 All these studies reported less phenol conversion and DIPP selectivity, more catalyst deactivation, and less catalyst stability.
In view of the studies mentioned above,3,7–15 there has been no report on use of H-beta and H-mordenite catalysts for vapour phase isopropylation of phenol using IPA as an alkylating agent to selective synthesis of DIPP (Table 1). The use of heterogeneous solid acid catalysts may be a very promising method for the synthesis of propofol based on their activity, selectivity, and re-usability and the increasing demand for ecologically and environmentally harmonized routes in the chemical industry.
Table 1 Comparison of isopropylation of phenol achieved with various alkylating agents over different catalysts
| Substrate |
Alkylating agent |
Process |
Catalyst |
Phenol conversion |
Propofol/DIPPselectivity |
Reference |
| Phenol |
Propylene |
Continuous |
Zeolites (Y, BEA, MOR, ZSM-5, TNU-9 and ZSM-11) |
2–10% |
<5% |
Xu et al.3 |
| Phenol |
Propylene |
Continuous |
H-β |
80% |
30% |
Wang et al.7 |
| Phenol |
Propylene |
Continuous |
H-USY |
71% |
31% |
Wang et al.7 |
| Phenol |
Isopropyl acetate |
Continuous |
Fe–Al-MCM-41 (50) |
76.8% |
13% |
Savidha et al.9 |
| Phenol |
IPA |
Batch |
Cs2.5H0.5PW12O40/K-10 |
50% |
16% |
Yadav & Salgaonkar11 |
| Phenol |
IPA |
Continuous |
Al2O3 |
100% |
52% |
Klemm & Taylor12 |
| Phenol |
IPA |
Continuous |
H-mordenite |
68% |
43% |
Present study |
| Phenol |
IPA |
Continuous |
H-beta |
94% |
56% |
Present study |
Thus, we are presenting maybe for the first time the use of H-beta and H-mordenite for vapour phase isopropylation of phenol (a model compound representing species in bio-oils) with IPA as an alkylating agent. The present study involves optimization of process parameters such as molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream in order to achieve higher phenol conversion and selectivity towards propofol (DIPP). A kinetic model is also proposed and validated with experimental data.
IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream in order to achieve higher phenol conversion and selectivity towards propofol (DIPP). A kinetic model is also proposed and validated with experimental data.
2 Results and discussion
2.1 Catalyst performance
X-ray diffraction patterns of synthesized catalysts H-beta and H-mordenite have shown the characteristic peaks of the zeolites with no contribution due to other crystalline or amorphous phases (Fig. 1).17,18 The characteristic peaks of H-beta that appeared in the 2θ ranges of 6.44°–9.44°, 19.32°–24.42°, 26.30°–27.52°, 32.72°–34.16° and 42.84°–44.82° confirmed the fully crystalline pure H-beta zeolitic phase (Fig. 1).17 Characteristic fingerprints of H-mordenite were observed in the 2θ ranges of 9.42°–9.85°, 13.5°–14.01°, 15.21°–15.74°, 19.14°–20.13°, 21.83°–22.32°, 25.28°–26.75°, 27.01°–28.71°, 30.42°–31.41°, 35.11°–36.07°, 43.92°–44.93° and 47.61°–49.59°, which confirmed phase purity and crystallinity.18 The obtained XRD patterns agreed well with those reported in the literature.17,18 Initially, isopropylation of phenol over thermal (without catalyst), H-beta and H-mordenite was carried out at the following reaction conditions: molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, WHSV of 4 h−1 and reaction temperature of 473 K. All the experiments were performed in triplicate and had 3% error as depicted by the error bars in the relevant figures. The time courses of the phenol conversion and selectivity towards DIPP are shown as Fig. 2. The phenol conversion obtained at 240 min followed the trend of H-beta (62%) > H-mordenite (54%) > thermal (without catalyst) (22%). Higher surface area and higher micropore volume (Vmicro) along with much smaller crystal size may be the decisive reasons for the higher activity of H-beta compared to H-mordenite (Table 2). In addition, selectivity towards DIPP over H-beta (46%) was higher than over H-mordenite (30%), which is attributed to the greater pore size of H-beta (7.5 Å) compared to H-mordenite (6.5 Å). Hence, the detailed optimization of process parameters was carried out for the isopropylation of phenol with IPA over H-beta to maximize the phenol conversion and DIPP selectivity. The comparative performance of H-mordenite at optimized process parameters is also discussed later.
2, WHSV of 4 h−1 and reaction temperature of 473 K. All the experiments were performed in triplicate and had 3% error as depicted by the error bars in the relevant figures. The time courses of the phenol conversion and selectivity towards DIPP are shown as Fig. 2. The phenol conversion obtained at 240 min followed the trend of H-beta (62%) > H-mordenite (54%) > thermal (without catalyst) (22%). Higher surface area and higher micropore volume (Vmicro) along with much smaller crystal size may be the decisive reasons for the higher activity of H-beta compared to H-mordenite (Table 2). In addition, selectivity towards DIPP over H-beta (46%) was higher than over H-mordenite (30%), which is attributed to the greater pore size of H-beta (7.5 Å) compared to H-mordenite (6.5 Å). Hence, the detailed optimization of process parameters was carried out for the isopropylation of phenol with IPA over H-beta to maximize the phenol conversion and DIPP selectivity. The comparative performance of H-mordenite at optimized process parameters is also discussed later.
 |
| | Fig. 1 X-ray diffraction patterns of H-beta and H-mordenite. | |
 |
| | Fig. 2 Isopropylation of phenol with IPA over thermal, H-beta and H-mordenite at the following reaction conditions: weight of catalyst of 1 g; molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1 IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; WHSV of 4 h−1 and reaction temperature of 473 K. 2; WHSV of 4 h−1 and reaction temperature of 473 K. | |
Table 2 Physicochemical properties of catalysts
| Catalyst |
Si/Al (bulk) |
BET surface area (m2 g−1) |
Crystal size (μm) |
Vmicro (cc N2 g−1) |
Total acidity (mmol g−1) |
| H-beta |
10 |
535 |
0.2–0.3 |
0.221 |
0.51 |
| H-mordenite |
10 |
426 |
1–2 |
0.188 |
1.28 |
2.2 Optimization of process parameters
2.2.1 Effect of molar ratio. Isopropylation of phenol with IPA was carried out by varying the molar ratio of phenol to IPA from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 over H-beta to assess its effect on the catalytic activity and selectivity. All the experiments were conducted in a kinetically controlled regime. The following hypotheses were used for the reaction: the rate of an alkylation is not controlled by the dehydration rate and the reaction follows adsorption of phenol and IPA on two different sites, which is discussed later. The time courses of the phenol conversion and selectivity towards DIPP over H-beta catalyst at different molar ratios are shown as Fig. 3. The phenol conversion and selectivity towards DIPP increased with an increase in the molar ratio from 1
6 over H-beta to assess its effect on the catalytic activity and selectivity. All the experiments were conducted in a kinetically controlled regime. The following hypotheses were used for the reaction: the rate of an alkylation is not controlled by the dehydration rate and the reaction follows adsorption of phenol and IPA on two different sites, which is discussed later. The time courses of the phenol conversion and selectivity towards DIPP over H-beta catalyst at different molar ratios are shown as Fig. 3. The phenol conversion and selectivity towards DIPP increased with an increase in the molar ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The maximum values of phenol conversion (72%) and selectivity towards DIPP (50%) were obtained at a molar ratio (phenol
4. The maximum values of phenol conversion (72%) and selectivity towards DIPP (50%) were obtained at a molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and reaction time of 240 min. This may be because at the lower concentration of phenol, more active sites of catalyst would be available for the surface adsorption of IPA, which in turn, results in quantitatively large formation of propylene and diisopropyl ether (DIPE) to react with phenol and also favours formation of DIPP.9–12 However, at ratios above 1
4 and reaction time of 240 min. This may be because at the lower concentration of phenol, more active sites of catalyst would be available for the surface adsorption of IPA, which in turn, results in quantitatively large formation of propylene and diisopropyl ether (DIPE) to react with phenol and also favours formation of DIPP.9–12 However, at ratios above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (phenol
4 (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPP), the phenol conversion (73%) and selectivity towards DIPP (51%) were marginally increased. Hence, the optimal phenol to IPA molar ratio of 1
IPP), the phenol conversion (73%) and selectivity towards DIPP (51%) were marginally increased. Hence, the optimal phenol to IPA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was used in further experiments.
4 was used in further experiments.
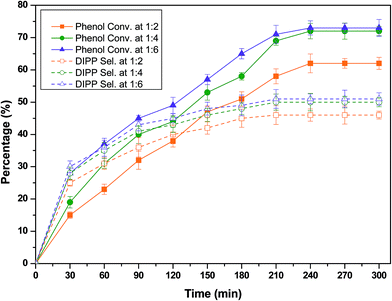 |
| | Fig. 3 Effect of molar ratio on the isopropylation of phenol with IPA over H-beta at the following reaction conditions: weight of catalyst of 1 g; WHSV of 4 h−1 and reaction temperature of 473 K. | |
2.2.3 Effect of reaction temperature. The influence of reaction temperature is very essential, as kinetic parameters, such as rate of reaction and activation energy, are directly dependent on variation in temperature. Fig. 5 depicts the effect of temperature on the conversion and selectivity of the isopropylation reaction of phenol with IPA as an alkylating agent over H-beta. The steady rise in the phenol conversion profile with a rise in temperature from 453 to 533 K was observed. The H-beta catalyst was observed to be stable in this temperature range. The phenol conversion was found to be increased from 66% to 93% with a rise in temperature from 453 to 533 K. This may be due to rise in temperature accelerating the rate of reaction. The selectivity towards DIPP was also observed to be increased from 52% to 56% with an increase in temperature from 473 to 533 K. The increase in DIPP selectivity suggests that its own rate of alkylation was much higher than the rate of formation of isopropylphenol from direct alkylation of phenol and from the rearrangement of isopropyl phenyl ether. With a further increase in temperature from 553 to 573 K, the phenol conversion was found to be decreased from 93% to 66%. The obtained decline in phenol conversion at higher temperature may be attributed to deisopropylation of isopropyl phenol (the product) into lower hydrocarbons and phenol.11 The selectivity of DIPP was observed to be decreased with an increase in temperature above 533 K. Hence, the temperature of 533 K was chosen as an optimum temperature and was used in further experiments. The optimum process parameters for the isopropylation of phenol were a molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, a WHSV of 2 h−1 and a temperature of 533 K. The comparative performance of H-mordenite was also evaluated at the optimized process parameters. The maximum phenol conversion of 68% and DIPP selectivity of 43% were obtained over H-mordenite.
4, a WHSV of 2 h−1 and a temperature of 533 K. The comparative performance of H-mordenite was also evaluated at the optimized process parameters. The maximum phenol conversion of 68% and DIPP selectivity of 43% were obtained over H-mordenite.
 |
| | Fig. 5 Effect of reaction temperature and time on the isopropylation of phenol with IPA over H-beta at the following reaction conditions: weight of catalyst of 1 g; molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1 IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; and WHSV of 2 h−1. 4; and WHSV of 2 h−1. | |
2.2.4 Effect of time on stream (TOS) and stability of catalyst. The stability of H-beta catalyst in the isopropylation of phenol with IPA as an alkylating agent was evaluated at the optimized process parameters: a molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, a WHSV of 2 h−1 and a temperature of 533 K. The catalyst was observed to stable for more than 25 h with stable phenol conversion of 93% and selectivity towards DIPP of 56% (Fig. 6). This may be due to the vapour phase isopropylation reaction delay the coke formation on the catalyst surface. After 25 h, the conversion of phenol was observed to be decreased to 87%. The catalyst was regenerated in situ by calcination at 823 K for 5 h in presence of air to remove the organic coke deposited on active sites. The regenerated catalyst was observed to regain its catalytic activity at 93% phenol conversion and 56% DIPP selectivity.
4, a WHSV of 2 h−1 and a temperature of 533 K. The catalyst was observed to stable for more than 25 h with stable phenol conversion of 93% and selectivity towards DIPP of 56% (Fig. 6). This may be due to the vapour phase isopropylation reaction delay the coke formation on the catalyst surface. After 25 h, the conversion of phenol was observed to be decreased to 87%. The catalyst was regenerated in situ by calcination at 823 K for 5 h in presence of air to remove the organic coke deposited on active sites. The regenerated catalyst was observed to regain its catalytic activity at 93% phenol conversion and 56% DIPP selectivity.
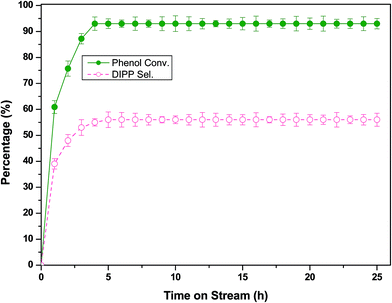 |
| | Fig. 6 Time on stream study of the isopropylation of phenol with IPA over H-beta at the following reaction conditions: weight of catalyst of 1 g; molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1 IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; WHSV of 2 h−1; and reaction temperature of 533 K. 4; WHSV of 2 h−1; and reaction temperature of 533 K. | |
2.3 Kinetic modeling and estimation of kinetic parameters
A model built on the hypothesis of two catalytic sites, S1 and S2, is proposed.11 In the studied temperature range (453–533 K), the alkylation rate was not monitored by the rate of dehydration, but the reaction mechanism involved a dual site mechanism including adsorption of reactants on two different sites, and then surface reaction of adsorbed reactant species to give the desired product. In the present case, the isopropylation of phenol (P) adsorbed on site S2 with IPA (I) adsorbed on the adjacent site S1 to give the 2,6-diisopropylphenol (propofol - D) as desired product and water (W) as byproduct, which is formed due to the following surface reaction as shown below:| |
 | (1) |
| |
 | (2) |
| |
 | (3) |
The site balance is given as follows,
| | |
CT − S1 = CV − S1 + CI − S1 + CW − S1
| (4) |
| | |
CT − S2 = CV − S2 + CP − S2 + CD − S2
| (5) |
The following adsorption equilibriums for different species are observed:
| |
 | (6) |
| |
 | (7) |
Thus, the rate of reaction of phenol is as follows:
| |
 | (8) |
With weak adsorption of all species, it gives the following:
where
| | |
kP = kSRCT − S1CT − S2K1−IK2 − P
| (10) |
Writing in terms of conversion and further integration results in,
| |
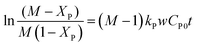 | (11) |
where
M is the molar ratio (phenol
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
IPA),
Xp is the fractional conversion of phenol,
kp is the rate constant of the isopropylation of phenol,
w is the weight of catalyst,
Cp0 is the initial concentration of phenol and
t is the reaction time.
Eqn (11) is an expression of a second-order reaction. The reaction rate constants at different temperatures can be obtained from a linear plot of ln((M − XP)/M(1 − XP)) versus (M − 1)CP0t. Fig. 7 shows a linear relationship between ln((M − XP)/M(1 − XP)) and (M − 1)CP0t at different reaction temperatures (453–533 K). Both reaction rate constants and R2 values of trend lines at different reaction temperature were obtained by linear regression (R2 > 0.98) using the software OriginPro70 (Table 3). The rate constant was found to be increased with an increase in reaction temperature. Moreover, the straight lines of the plots fitted the experimental data well (Fig. 7). This is a clear indication that the developed kinetic model is valid for this reaction.
 |
| | Fig. 7 Kinetic model plot of the isopropylation of phenol with IPA over H-beta to obtain the reaction rate constants at different temperatures. | |
Table 3 Reaction rate constants (kp) and R2 values of trend lines for the isopropylation of phenol with IPA over H-beta at different temperatures
| Temperature (K) |
453 |
473 |
493 |
513 |
533 |
| kp × 10−3(L g−1 min−1) |
1.74 |
2.19 |
2.91 |
3.69 |
4.76 |
| R2 |
0.9939 |
0.9932 |
0.9875 |
0.9964 |
0.9899 |
To consider the effect of reaction temperature on the kinetic model, the Arrhenius equation can be written as follows:
| |
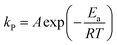 | (12) |
where
R is the gas constant (8.314 J mol
−1 K
−1).
The plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kp can be used as a function of the reciprocal temperature,
kp can be used as a function of the reciprocal temperature,
| |
 | (13) |
The plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kp versus 1/T for the isopropylation of phenol with IPA over H-beta is represented by a straight line (Fig. 8). Both the pre-exponential factor (A) and activation energy (Ea) were obtained by linear regression (R2 > 0.99) using the software OriginPro70. The activation energy (Ea) and pre-exponential factor (A) for isopropylation of phenol with IPA over H-beta were 25.39 kJ mol−1 and 1.4375 L min−1 mol−1, respectively. This indicates that the isopropylation reaction was in the kinetic regime owing to the high activation energy.
kp versus 1/T for the isopropylation of phenol with IPA over H-beta is represented by a straight line (Fig. 8). Both the pre-exponential factor (A) and activation energy (Ea) were obtained by linear regression (R2 > 0.99) using the software OriginPro70. The activation energy (Ea) and pre-exponential factor (A) for isopropylation of phenol with IPA over H-beta were 25.39 kJ mol−1 and 1.4375 L min−1 mol−1, respectively. This indicates that the isopropylation reaction was in the kinetic regime owing to the high activation energy.
 |
| | Fig. 8 Arrhenius plot of the isopropylation of phenol with IPA over H-beta to obtain the activation energy and pre-exponential factor. | |
2.4 Merits of the present method
Xu et al.3 reported gas phase alkylation of phenol with propylene over various zeolites such as Y, BEA, MOR, ZSM-5, TNU-9 and ZSM-11 and obtained phenol conversions of 2–10% and selectivity towards DIPP of <5%. Wang et al.7 studied C alkylation of phenol with propylene over H-β and USY and obtained phenol conversions of 80% and 71% and selectivities towards DIPP of 30% and 31%, respectively. They have also reported the vapour phase alkylation of phenol with propylene using HZSM5 by varying the Si/Al ratio and introducing Cs+ sites via ion exchange Cs+ and reported a 50% decrease in the original selectivity.8 Savidha et al.9,10 reported the vapour phase alkylation of phenol with isopropyl acetate catalysed by Zn–Al-MCM-41, Fe–Al-MCM-41 and Al-MCM-41. They obtained a maximum phenol conversion of 76.8% and 13% selectivity towards DIPP over Fe–Al-MCM-41 (50) catalyst.9,10 Yadav and Salgaonkar reported isopropylation of phenol with IPA over 20% (w/w) Cs2.5H0.5PW12O40/K-10 in a batch process and obtained the maximum phenol conversion of 50% with a selectivity towards DIPP of 16%.11 Klemm and Taylor reported 100% phenol conversion with 52% DIPP selectivity over Al3O3 catalyst.12
Table 1 describes the merits of the present method. All of the reported studies referenced in Table 1 have some limitations in terms of lower activity value, less catalyst stability and the use of an alkylating agent such as propylene or isopropyl acetate, which leads to fast deactivation by coking. In order to eliminate these limitations, the use of IPA as an alkylating agent and an improved version of the catalyst will be advantageous. The present study was carried out using IPA as an alkylating agent in continuous vapour phase over H-mordenite and H-beta, which has not been reported to date. In this context, the present method of using H-mordenite and H-beta for the synthesis of propofol (2,6-isopropyl phenol/DIPP) by isopropylation of phenol (a model compound representing species in bio-oils) with IPA offers a greener methodology with potential advantages with respect to a higher phenol conversion of 94% and a selectivity towards propofol of 56% over H-beta, which is much higher than that the reported at milder operating parameters.3,7–15 A maximum phenol conversion of 68% and DIPP selectivity of 43% was obtained over H-mordenite at optimized process parameters. H-beta catalyst was also observed to be highly active and stable for 25 h. This study demonstrates the principles of green chemistry such as a safe synthetic method, reusable heterogeneous catalyst, ambient operating parameters and minimized material diversity (high selectivity of desired product).20 This study provides an efficient catalytic process to stabilize bio-oils containing phenolic compounds and to synthesize more valuable products such as propofol. The scale-up work is an on-going activity and will be considered in future publications. This study opens an avenue for the development of an eco-friendly catalytic process for the selective synthesis of propofol (DIPP), a widely used intravenous general anaesthetic.
3 Conclusions
Selective synthesis of propofol (2,6-isopropyl phenol/DIPP) by isopropylation of phenol using isopropyl alcohol (IPA) as an alkylating agent over the zeolites H-beta and H-mordenite was carried out, probably for the first time. H-beta was observed to be the optimum catalyst over H-mordenite. A maximum phenol conversion of 94% with 56% selectivity towards propofol was achieved over H-beta, which is far better than reported values. H-beta catalyst was also observed to be highly active and stable for 25 h. A second order kinetic model is proposed and validated with experimental data with an R2 > 0.98. The activation energy is calculated to be 25.39 kJ mol−1. This indicates that the isopropylation reaction is kinetically controlled owing to the high activation energy. Thus, isopropylation of phenol (a model compound representing species in bio-oils) over H-beta can be considered an environmental benign catalytic process to stabilize bio-oils and make products that are more valuable. This study opens an avenue for the development of an eco-friendly catalytic process for the selective synthesis of propofol.
4 Experimental section
4.1 Materials
Phenol and isopropyl alcohol (IPA) were obtained from E. Merck Ltd., Mumbai, India. All reagents used were of analytical quality and used as received.
4.2 Catalyst synthesis and characterization
The protonic forms of zeolite catalysts H-beta and H-mordenite with Si/Al ratio of 10 were synthesized according to the reported procedures.17,18 The crystallinity and phase purity of the synthesized samples were confirmed by powder X-ray diffraction (XRD) patterns using an X-ray diffractometer (Rigaku Miniflex, Tokyo, Japan). The XRD patterns were recorded on an X-ray diffractometer (P Analytical PXRD system, Model X-Pert PRO-1712) using CuK∝ radiation at a scanning rate of 0.0671 s−1 in the 2θ ranging from 5° to 60° (Fig. 1). The specific surface area and micropore volume (Vmicro) of catalysts were obtained from nitrogen adsorption–desorption isotherms measured in an SA 3100 analyser (Beckman Coulter, CA, USA). The adsorption was carried out at 77 K overnight under nitrogen with a residual vapour pressure of 0.3 Pa. Temperature-programmed ammonia desorption (TPAD) was used to determine the total acidity of all synthesized samples (Table 2). TPAD was performed using a Micromeritics AutoChem 2910 (Norcross, GA, USA). Briefly, 0.5 g of sample was loaded and activated at 873 K in a quartz cell, in the presence of He flow (20 mL min−1) for 2 h. Pre-saturation was accomplished by passing 10% ammonia in He for 1 h at ambient temperature (303 K). Then, the sample was flushed with helium for 1 h at 323 K to remove excess ammonia. The adsorbed ammonia was desorbed in helium flow (30 mL min−1) with a heating rate of 10 K min−1 as a function of temperature from 323 to 773 K. Thermal conductive detector (TCD) detected the desorbed ammonia.
4.3 Catalyst evaluation and analysis
Isopropylation of phenol with IPA over protonic forms of H-beta and H-mordenite zeolite samples were carried out in a continuous, down–flow, fixed bed reactor SS316 (40 cm length × 2 cm internal diameter) at atmospheric pressure. The amount of 1.0 g (2 cm height) of 10–20 mesh granules of self-bonded catalyst was loaded in the reactor. Prior to reaction, the activation of catalysts was carried out at 723 K for 8 h in air to drive off adsorbed hydrocarbon and moisture, if any. Reactants (phenol and IPA) were fed through a syringe pump (ISCO, USA) into the reactor at the desired reaction temperature. The analysis of products was performed using a Shimadzu gas chromatograph (Model GC 15A), coupled with an Apiezome L (B.P. 1/1) column and a flame ionization detector. The column used was of 0.0032 m i.d. × 50 m length. The conversion of phenol and selectivity towards propofol (DIPP) as a function of time on stream over H-beta catalysts was investigated at temperatures of 453–573 K with a weight hourly space velocity (WHSV) of 2–6 h−1 and feed (phenol:IPA) molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.
6.
Phenol conversion was calculated according to eqn (14):
| |
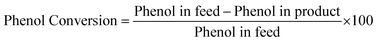 | (14) |
Product selectivity was calculated according to eqn (15):
| |
 | (15) |
Nomenclature
| A | Pre-exponential factor (L min−1 mol−1) |
| Ci | Concentration of species i (mol L−1) |
| CT | Concentration of total sites of catalyst (mol L−1) |
| CV | Concentration of vacant sites of catalyst (mol L−1) |
| D | 2,6-Diisopropylphenol/DIPP/propofol |
| E | Diisopropyl ether/DIPE |
| Ea | Activation energy (J mol−1) |
| I | IPA/Isopropyl alcohol |
| i–j | Species ‘j’ adsorbed on site ‘i’ |
| IPP | Isopropylphenol |
| K | Adsorption/reaction rate constant |
| kp | Reaction rate constants of isopropylation of phenol (g mol−1 min−1) |
| M | Molar ratio (phenol:IPA) |
| P | Phenol |
| S | Vacant catalyst sites |
| SR | Surface reaction |
| t | Time (min) |
| T | Temperature (K) |
| T-Si | Total sites S of type ‘i’ |
| V-Si | Vacant sites S of type ‘i’ |
| w | Weight of catalyst (g) |
| W | Water |
| Xp | Fractional conversion of phenol |
Acknowledgements
The authors thank Dr Prashant S. Niphadkar from CSIR-NCL, Pune (India) for editing the manuscript.
References
- C. C. Liao and T. W. Chung, Chem. Eng. Res. Des., 2013, 91, 2457–2464 CrossRef CAS PubMed.
- A. H. Jadhav, H. Kim and I. T. Hwang, Bioresour. Technol., 2013, 132, 342–350 CrossRef CAS PubMed.
- W. Xu, S. J. Miller, P. K. Agrawal and C. W. Jones, Appl. Catal., A, 2013, 459, 114–120 CrossRef CAS PubMed.
- D. Mohan, C. U. Pittman and P. H. Steele, Energy Fuels, 2006, 20, 848–889 CrossRef CAS.
- W. Xu, S. J. Miller, P. K. Agrawal and C. W. Jones, ChemSusChem, 2012, 5, 667–675 CrossRef CAS PubMed.
- Z. Zhang, Q. Wang, P. Tripathi, Jr and C. U. Pittman, Green Chem., 2011, 13, 940–949 RSC.
- B. Wang, C. W. Lee, T. Cai and S. Park, Stud. Surf. Sci. Catal., 2001, 135, 4121–4128 CAS.
- B. Wang, C. W. Lee, T. Cai and S. Park, Catal. Lett., 2011, 76, 219–224 CrossRef.
- R. Savidha, A. Pandurangan, M. Palaichamy and V. Murugesan, Catal. Lett., 2003, 91, 49–61 CrossRef CAS.
- R. Savidha and A. Pandurangan, Appl. Catal., A, 2004, 262, 1–11 CrossRef CAS PubMed.
- G. D. Yadav and S. S. Salgaonkar, Ind. Eng. Chem. Res., 2005, 44, 1706–1715 CrossRef CAS.
- L. H. Klemm and D. R. Taylor, J. Org. Chem., 1980, 45, 4320–4329 CrossRef CAS.
- L. Wei, Y. Shang and P. Yang, React. Kinet. Catal. Lett., 2008, 93, 265–271 CrossRef CAS.
- D. Wang, X. Li, Z. Liu, Y. Zhang, Z. Xie and Y. Tang, J. Colloid Interface Sci., 2010, 350, 290–294 CrossRef CAS PubMed.
- L. Wang, X. C. Yang, L. Zhang, Y. Gao, Y. Wang and Y. Shang, React. Kinet., Mech. Catal., 2013, 109, 213–220 CrossRef CAS.
- G. M. S. Yip, Z. W. Chen, C. J. Edge, E. H. Smith, R. Dickinson, E. Hohenester, R. R. Townsend, K. Fuchs, W. Sieghart, A. S. Evers and N. P. Franks, Nat. Chem. Biol., 2013, 9, 715–720 CrossRef CAS PubMed.
- M. W. Kasture, P. S. Niphadkar, N. Sharanappa, S. P. Mirajkar, V. V. Bokade and P. N. Joshi, J. Catal., 2004, 227, 375–383 CrossRef CAS PubMed.
- A. Shaikh, P. N. Joshi, N. E. Jacob and V. P. Shiralkar, Zeolites, 1993, 13, 511–517 CrossRef CAS.
- C. Perego and S. Peratello, Catal. Today, 1999, 52, 133–145 CrossRef CAS.
- S. Y. Tang, R. A. Bourne, L. Richard, R. L. Smith and M. Poliakoff, Green Chem., 2008, 10, 268–269 RSC.
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream were performed. H-beta (94% phenol conv. and 56% DIPP sel.) was found to be a potential and more active catalyst than H-mordenite (68% phenol conv. and 43% DIPP sel.) at optimized process parameters. A kinetic model is proposed to probe the intricate reaction kinetics and validated (R2 > 0.98) by the experimental results. H-beta catalyst was observed to be stable for more than 25 h with 94% phenol conversion and 56% selectivity towards DIPP at optimized process parameters. The phenol conversion and DIPP selectivity obtained in the present study are higher than those reported so far. The activation energy obtained for isopropylation of phenol with IPA over H-beta is calculated to be 25.39 kJ mol−1.
IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream were performed. H-beta (94% phenol conv. and 56% DIPP sel.) was found to be a potential and more active catalyst than H-mordenite (68% phenol conv. and 43% DIPP sel.) at optimized process parameters. A kinetic model is proposed to probe the intricate reaction kinetics and validated (R2 > 0.98) by the experimental results. H-beta catalyst was observed to be stable for more than 25 h with 94% phenol conversion and 56% selectivity towards DIPP at optimized process parameters. The phenol conversion and DIPP selectivity obtained in the present study are higher than those reported so far. The activation energy obtained for isopropylation of phenol with IPA over H-beta is calculated to be 25.39 kJ mol−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream in order to achieve higher phenol conversion and selectivity towards propofol (DIPP). A kinetic model is also proposed and validated with experimental data.
IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream in order to achieve higher phenol conversion and selectivity towards propofol (DIPP). A kinetic model is also proposed and validated with experimental data.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, WHSV of 4 h−1 and reaction temperature of 473 K. All the experiments were performed in triplicate and had 3% error as depicted by the error bars in the relevant figures. The time courses of the phenol conversion and selectivity towards DIPP are shown as Fig. 2. The phenol conversion obtained at 240 min followed the trend of H-beta (62%) > H-mordenite (54%) > thermal (without catalyst) (22%). Higher surface area and higher micropore volume (Vmicro) along with much smaller crystal size may be the decisive reasons for the higher activity of H-beta compared to H-mordenite (Table 2). In addition, selectivity towards DIPP over H-beta (46%) was higher than over H-mordenite (30%), which is attributed to the greater pore size of H-beta (7.5 Å) compared to H-mordenite (6.5 Å). Hence, the detailed optimization of process parameters was carried out for the isopropylation of phenol with IPA over H-beta to maximize the phenol conversion and DIPP selectivity. The comparative performance of H-mordenite at optimized process parameters is also discussed later.
2, WHSV of 4 h−1 and reaction temperature of 473 K. All the experiments were performed in triplicate and had 3% error as depicted by the error bars in the relevant figures. The time courses of the phenol conversion and selectivity towards DIPP are shown as Fig. 2. The phenol conversion obtained at 240 min followed the trend of H-beta (62%) > H-mordenite (54%) > thermal (without catalyst) (22%). Higher surface area and higher micropore volume (Vmicro) along with much smaller crystal size may be the decisive reasons for the higher activity of H-beta compared to H-mordenite (Table 2). In addition, selectivity towards DIPP over H-beta (46%) was higher than over H-mordenite (30%), which is attributed to the greater pore size of H-beta (7.5 Å) compared to H-mordenite (6.5 Å). Hence, the detailed optimization of process parameters was carried out for the isopropylation of phenol with IPA over H-beta to maximize the phenol conversion and DIPP selectivity. The comparative performance of H-mordenite at optimized process parameters is also discussed later.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 over H-beta to assess its effect on the catalytic activity and selectivity. All the experiments were conducted in a kinetically controlled regime. The following hypotheses were used for the reaction: the rate of an alkylation is not controlled by the dehydration rate and the reaction follows adsorption of phenol and IPA on two different sites, which is discussed later. The time courses of the phenol conversion and selectivity towards DIPP over H-beta catalyst at different molar ratios are shown as Fig. 3. The phenol conversion and selectivity towards DIPP increased with an increase in the molar ratio from 1
6 over H-beta to assess its effect on the catalytic activity and selectivity. All the experiments were conducted in a kinetically controlled regime. The following hypotheses were used for the reaction: the rate of an alkylation is not controlled by the dehydration rate and the reaction follows adsorption of phenol and IPA on two different sites, which is discussed later. The time courses of the phenol conversion and selectivity towards DIPP over H-beta catalyst at different molar ratios are shown as Fig. 3. The phenol conversion and selectivity towards DIPP increased with an increase in the molar ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The maximum values of phenol conversion (72%) and selectivity towards DIPP (50%) were obtained at a molar ratio (phenol
4. The maximum values of phenol conversion (72%) and selectivity towards DIPP (50%) were obtained at a molar ratio (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and reaction time of 240 min. This may be because at the lower concentration of phenol, more active sites of catalyst would be available for the surface adsorption of IPA, which in turn, results in quantitatively large formation of propylene and diisopropyl ether (DIPE) to react with phenol and also favours formation of DIPP.9–12 However, at ratios above 1
4 and reaction time of 240 min. This may be because at the lower concentration of phenol, more active sites of catalyst would be available for the surface adsorption of IPA, which in turn, results in quantitatively large formation of propylene and diisopropyl ether (DIPE) to react with phenol and also favours formation of DIPP.9–12 However, at ratios above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (phenol
4 (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPP), the phenol conversion (73%) and selectivity towards DIPP (51%) were marginally increased. Hence, the optimal phenol to IPA molar ratio of 1
IPP), the phenol conversion (73%) and selectivity towards DIPP (51%) were marginally increased. Hence, the optimal phenol to IPA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was used in further experiments.
4 was used in further experiments.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, a WHSV of 2 h−1 and a temperature of 533 K. The comparative performance of H-mordenite was also evaluated at the optimized process parameters. The maximum phenol conversion of 68% and DIPP selectivity of 43% were obtained over H-mordenite.
4, a WHSV of 2 h−1 and a temperature of 533 K. The comparative performance of H-mordenite was also evaluated at the optimized process parameters. The maximum phenol conversion of 68% and DIPP selectivity of 43% were obtained over H-mordenite.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA) of 1
IPA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, a WHSV of 2 h−1 and a temperature of 533 K. The catalyst was observed to stable for more than 25 h with stable phenol conversion of 93% and selectivity towards DIPP of 56% (Fig. 6). This may be due to the vapour phase isopropylation reaction delay the coke formation on the catalyst surface. After 25 h, the conversion of phenol was observed to be decreased to 87%. The catalyst was regenerated in situ by calcination at 823 K for 5 h in presence of air to remove the organic coke deposited on active sites. The regenerated catalyst was observed to regain its catalytic activity at 93% phenol conversion and 56% DIPP selectivity.
4, a WHSV of 2 h−1 and a temperature of 533 K. The catalyst was observed to stable for more than 25 h with stable phenol conversion of 93% and selectivity towards DIPP of 56% (Fig. 6). This may be due to the vapour phase isopropylation reaction delay the coke formation on the catalyst surface. After 25 h, the conversion of phenol was observed to be decreased to 87%. The catalyst was regenerated in situ by calcination at 823 K for 5 h in presence of air to remove the organic coke deposited on active sites. The regenerated catalyst was observed to regain its catalytic activity at 93% phenol conversion and 56% DIPP selectivity.






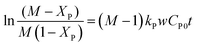
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA), Xp is the fractional conversion of phenol, kp is the rate constant of the isopropylation of phenol, w is the weight of catalyst, Cp0 is the initial concentration of phenol and t is the reaction time.
IPA), Xp is the fractional conversion of phenol, kp is the rate constant of the isopropylation of phenol, w is the weight of catalyst, Cp0 is the initial concentration of phenol and t is the reaction time.

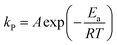
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kp can be used as a function of the reciprocal temperature,
kp can be used as a function of the reciprocal temperature,
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kp versus 1/T for the isopropylation of phenol with IPA over H-beta is represented by a straight line (Fig. 8). Both the pre-exponential factor (A) and activation energy (Ea) were obtained by linear regression (R2 > 0.99) using the software OriginPro70. The activation energy (Ea) and pre-exponential factor (A) for isopropylation of phenol with IPA over H-beta were 25.39 kJ mol−1 and 1.4375 L min−1 mol−1, respectively. This indicates that the isopropylation reaction was in the kinetic regime owing to the high activation energy.
kp versus 1/T for the isopropylation of phenol with IPA over H-beta is represented by a straight line (Fig. 8). Both the pre-exponential factor (A) and activation energy (Ea) were obtained by linear regression (R2 > 0.99) using the software OriginPro70. The activation energy (Ea) and pre-exponential factor (A) for isopropylation of phenol with IPA over H-beta were 25.39 kJ mol−1 and 1.4375 L min−1 mol−1, respectively. This indicates that the isopropylation reaction was in the kinetic regime owing to the high activation energy.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.
6.