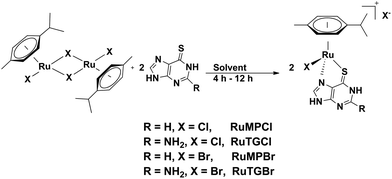
Mitigating UVA light induced reactivity of 6-thioguanine through formation of a Ru(II) half-sandwich complex†
Raja Mitra and
Ashoka G. Samuelson*
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560012, India. E-mail: ashoka@ipc.iisc.ernet.in; Fax: +91-80-23601552; Tel: +91-80-22932663
First published on 27th May 2014
Abstract
The organometallic complex of (η6-cymene)Ru(II)Br with 6-thioguanine (6-TG) shows better photostability than the biologically active 6-thioguanine which is used as an immunosuppressant and as an anticancer agent.
Leukemia is a dreaded cancer of the blood and bone marrow. Among the various drugs available for treating leukemia, 6-thioguanine (6-TG) has been the drug of choice since 1966, especially for treating acute lymphatic leukemia (ALL).1 Unfortunately 6-TG, which is also given as an immunosuppressant to patients who have undergone organ transplants, causes severe side effects. Karran and coworkers have skillfully pinned down the source of the problem to a reaction undergone by 6-TG on irradiation with UVA light.2 The major products formed are guanine-6-sulfonate (GSO3) and reactive oxygen species (ROS) resulting in skin cancer.3,4 Hence, ways and means of modulating the reactivity of 6-TG are of great interest.
Organometallic half-sandwich Ru(II) complexes are known to have significant anticancer activity.5–13 The complexes bind to the enzyme, transferrin, which is taken up selectively by cancer cells overexpressing transferrin receptors, thereby increasing internalization.14,15 So using 6-TG as an ancillary ligand for the half-sandwich Ru(II) moiety should result in photoreactivity modulation of 6-TG and deliver the complex to cancer cells through the transferrin enzyme. To this end, we synthesized and studied half-sandwich complexes of 6-TG and the related 6-mercaptopurine (6-MP).
Half-sandwich η6-arene ruthenium complexes of thiopurine as an ancillary ligand are rare.16 We synthesize the target complexes through a convenient reaction of the ruthenium(II) dimer [(η6-cymene)RuX2]2 (X = Cl or, Br) with thiopurines in a suitable solvent. The chloro complexes of Ru(II) with thiopurine precipitate from the reaction mixture in dichloromethane during the course of the reaction in reasonable yields. In the case of the bromido complexes, the reaction was carried out in methanol and pure complexes were precipitated by addition of diethyl ether (Scheme 1). The complexes are characterized by standard spectroscopic techniques and analytical data. Three of the complexes are also amenable to single crystal X-ray structure analysis. Single crystals are obtained by slow diffusion of diethyl ether into a solution of the complexes in DMF. Based on the structural and 1H NMR data, it is clear that the complexes have the expected half-sandwich structure, with the ancillary ligand coordinating through S and N in κ2 fashion resulting in dissociation of X− which is present in the lattice as a hydrogen-bond stabilized counter ion.
All bond distances, Ru–S, Ru–N and those between the cymene and Ru are as expected.11,17 The important bond lengths and bond angles for the complexes are listed in Tables S1 and S2.† Crystallographic data and refinement parameters for all complexes are listed in Table S3.†
The ORTEP representation of the molecular structures of the complexes is shown in Fig. 1. The lattice abounds in H bonding between the counter anion and ancillary ligands and in the case of RuTGCl, intermolecular H-bonding further stabilizes the lattice (Fig. S1†).
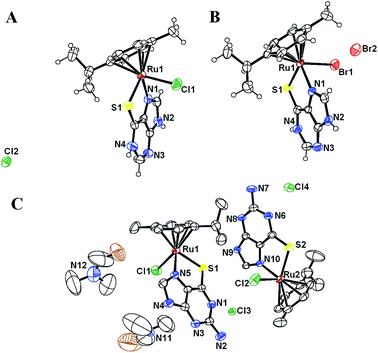 | ||
| Fig. 1 ORTEP view of RuMPCl (A), RuMPBr (B) and RuTGCl.DMF (C) at 40% thermal ellipsoid. Hydrogen atoms are omitted in structure (C) to improve clarity. | ||
1H NMR spectra of these complexes in dry d6-DMSO are completely consistent with the molecular structure (Fig. S2†). The thioguanine complex dissolved in D2O–H2O in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio showed very little change in water suggesting significant stability of the complexes in water (Fig. S3a†). However, 1H NMR spectra of RuMPCl or RuMPBr complexes reveal the formation of a mixture of species. However, it is difficult to decipher the nuclearity of the RuMPX complexes from the 1H NMR spectra (Fig. S3b†).
4 ratio showed very little change in water suggesting significant stability of the complexes in water (Fig. S3a†). However, 1H NMR spectra of RuMPCl or RuMPBr complexes reveal the formation of a mixture of species. However, it is difficult to decipher the nuclearity of the RuMPX complexes from the 1H NMR spectra (Fig. S3b†).
Further characterization of the complexes formed on reaction with water as a function of time was attempted using ESI-MS spectrometry (Table S5†). We expected the formation of an aqua complex as observed earlier for organometallic ruthenium complexes with ethylenediamine and 1,3,5-triaza-7-phosphatricyclo[3.3.1.1]decane, (PTA) ligands.17–19 However, ESI-MS analysis showed formation of a dimeric species and not the aqua complex (Fig. 2). The singly charged protonated dinuclear ruthenium species and a singly charged mononuclear species were the most abundant ions in the ESI-MS spectra (Fig. S4†). It is possible that the major species is dinuclear and it is fragmented during ESI-MS acquisition resulting in higher amount of mononuclear species. Based on these studies we suggest the following steps in the hydrolysis and dimerization similar to the reactions observed in the case of 2-mercaptobenzothiazole half-sandwich complexes with Ru(II)11 (Scheme 2).
Using a home built photoreactor, for which the luminosity was standardized with an actinometer,20 the photochemical behavior of the complexes and of 6-TG was studied (Fig. S5 and S6†). Aqueous solutions of the ruthenium complexes (0.1 mM) were irradiated for 10 min and the UV-Vis spectral changes were followed. Under these conditions, solutions of RuTGBr were quite stable whereas solutions of RuTGCl showed some decrease in its concentration (Fig. 3).
ESI-MS spectra of RuTGX complexes before and after photoirradiation showed the peak at m/z 402.03 corresponding to [(η6-cymene)Ru(6-TG-H)]+ to be the most abundant species, irrespective of the halide in the complex and a small peak at m/z 803.05 corresponding to a dinuclear species. After irradiation, the spectral features were mostly unchanged (Fig. S7†). To confirm the photostability of these complexes, 1H NMR studies were also carried out. Three sets of samples each containing 8 mg of RuTGCl or RuTGBr dissolved in 5 mL of water were irradiated separately 30 min, 10 min or kept in the dark. The samples were then lyophilized and 1H NMR spectrum of the residue recorded (Fig. S8†). 1H NMR spectra suggested that both complexes were quite stable i.e., no leaching of the ancillary ligand and the arene cap was observed after irradiation. Stability of RuTGBr in the presence of UVA light was further confirmed by using 4-hydroxy benzoic acid as an internal calibrant for 1H NMR spectra (Fig. S9†). No significant changes were observed after photoirradiation in the ratios of internal calibrant with cymene peaks of RuTGBr. Based on these experiments one can say that under conditions which cause 6-TG to undergo photooxidation, RuTGBr is quite stable. Leaching of the arene group was not observed contrary to earlier reports on some half-sandwich complexes.21
Having established the greater photostability and solution behavior of these complexes relative to 6-TG, it remained to be seen if they have better or equivalent anticancer activity. Growth inhibition (GI50) of all complexes and 6-TG were checked against K562 (chronic myelogenous leukemia), Jurkat (leukemic T cell lymphoblast) and Molt-4 (leukemic T cell lymphoblast) cell lines by the sulphorhodamine B (SRB) assay.22 The results are listed in Table 1. GI50 values suggest that all complexes are highly active against the K562 cell line. Cytotoxicity values of the complexes against the K562 cell line are comparable to the active drug, 6-thioguanine (6-TG) and 6-mercaptopurine (6-MP). In the Jurkat cell line, 6-MP complexes are inactive. However, 6-TG complexes are quite active. It was also observed that against the Molt-4 cell line, RuMPCl, RuTGCl and RuTGBr are more active than 6-TG. It was observed that all complexes have a negative log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value, and are quite soluble in water. So RuTGX complexes are suitable, like 6-TG, for oral administration to the patient.
P value, and are quite soluble in water. So RuTGX complexes are suitable, like 6-TG, for oral administration to the patient.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of [(η6-cymene)RuIIX(SN)] complexes
P values of [(η6-cymene)RuIIX(SN)] complexes
| Compounds | GI50 (μM, 48 h) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
||
|---|---|---|---|---|
| K562 | Jurkat | Molt-4 | ||
| RuMPCl | <0.1 | 34.7 | <0.1 | −1.45 ± 0.06 |
| RuTGCl | <0.1 | <0.1 | <0.1 | −0.63 ± 0.09 |
| RuMPBr | <0.1 | 42.2 | 32.1 | −0.90 ± 0.04 |
| RuTGBr | <0.1 | <0.1 | <0.1 | −1.42 ± 0.25 |
| 6-TG | <0.1 | <0.1 | 9.4 | — |
| 6-MP | <0.1 | <0.1 | <0.1 | — |
In conclusion, the aqueous solutions of RuTGBr, are stable in the presence of UVA light in contrast to 6-TG. RuTGCl and RuTGBr show significant anticancer activity against three leukemia cell lines. Clearly, the ruthenium complex of 6-TG (RuTGBr) is a potential alternative to the photosensitive 6-thioguanine as an immunosuppressant and in the treatment of various malignant leukemia.
Authors would like to thank DST, New Delhi, for generous funding. RM thanks CSIR, New Delhi for a Senior Research Fellowship. Authors would like to thank Dr A. Juvekar of ACTREC, Mumbai for growth inhibition data.
Notes and references
- P. Karran and N. Attard, Nat. Rev. Cancer, 2008, 8, 24–36 CrossRef CAS PubMed.
- P. O'Donovan, C. M. Perrett, X. Zhang, B. Montaner, Y.-Z. Xu, C. A. Harwood, J. M. McGregor, S. L. Walker, F. Hanaoka and P. Karran, Science, 2005, 309, 1871–1874 CrossRef PubMed.
- X. Ren, F. Li, G. Jeffs, X. Zhang, Y.-Z. Xu and P. Karran, Nucleic Acids Res., 2010, 38, 1832–1840 CrossRef CAS PubMed.
- X. Zou, H. Zhao, Y. Yu and H. Su, J. Am. Chem. Soc., 2013, 135, 4509–4515 CrossRef CAS PubMed.
- Y. K. Yan, M. Melchart, A. Habtemariam and P. J. Sadler, Chem. Commun., 2005, 4764–4776 RSC.
- N. P. E. Barry and P. J. Sadler, Chem. Commun., 2013, 49, 5106–5131 RSC.
- C. G. Hartinger and P. J. Dyson, Chem. Soc. Rev., 2009, 38, 391–401 RSC.
- G. Gasser, I. Ott and N. Metzler-Nolte, J. Med. Chem., 2010, 54, 3–25 CrossRef PubMed.
- A. Kurzwernhart, W. Kandioller, C. Bartel, S. Bachler, R. Trondl, G. Muhlgassner, M. A. Jakupec, V. B. Arion, D. Marko, B. K. Keppler and C. G. Hartinger, Chem. Commun., 2012, 48, 4839–4841 RSC.
- S. Das, S. Sinha, R. Britto, K. Somasundaram and A. G. Samuelson, J. Inorg. Biochem., 2010, 104, 93–104 CrossRef CAS PubMed.
- R. Mitra, S. Das, S. V. Shinde, S. Sinha, K. Somasundaram and A. G. Samuelson, Chem.–Eur. J., 2012, 18, 12278–12291 CrossRef CAS PubMed.
- I. Bratsos, E. Mitri, F. Ravalico, E. Zangrando, T. Gianferrara, A. Bergamo and E. Alessio, Dalton Trans., 2012, 41, 7358–7371 RSC.
- L. Leyva, C. Sirlin, L. Rubio, C. Franco, R. Le Lagadec, J. Spencer, P. Bischoff, C. Gaiddon, J.-P. Loeffler and M. Pfeffer, Eur. J. Inorg. Chem., 2007, 2007, 3055–3066 CrossRef.
- Z. M. Qian, H. Li, H. Sun and K. Ho, Pharmacol. Rev., 2002, 54, 561–587 CrossRef CAS.
- W. Guo, W. Zheng, Q. Luo, X. Li, Y. Zhao, S. Xiong and F. Wang, Inorg. Chem., 2013, 52, 5328–5338 CrossRef CAS PubMed.
- K. Yamanari, R. Ito, S. Yamamoto, T. Konno, A. Fuyuhiro, K. Fujioka and R. Arakawa, Inorg. Chem., 2002, 41, 6824–6830 CrossRef CAS PubMed.
- F. Wang, H. Chen, S. Parsons, I. D. H. Oswald, J. E. Davidson and P. J. Sadler, Chem.–Eur. J., 2003, 9, 5810–5820 CrossRef CAS PubMed.
- F. Wang, A. Habtemariam, E. P. van der Geer, R. Fernandez, M. Melchart, R. J. Deeth, R. Aird, S. Guichard, F. P. Fabbiani, P. Lozano-Casal, I. D. Oswald, D. I. Jodrell, S. Parsons and P. J. Sadler, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 18269–18274 CrossRef CAS PubMed.
- K. J. Kilpin, S. M. Cammack, C. M. Clavel and P. J. Dyson, Dalton Trans., 2013, 42, 2008–2014 RSC.
- T. Lehóczki, É. Józsa and K. Ősz, J. Photochem. Photobiol., A, 2013, 251, 63–68 CrossRef PubMed.
- W. Weber and P. C. Ford, Inorg. Chem., 1986, 25, 1088–1092 CrossRef CAS.
- N. J. M. Sanghamitra, M. K. Adwankar, A. S. Juvekar, V. Khurajjam, C. Wycliff and A. G. Samuelson, Indian J. Chem., 2011, 50A, 465–473 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, 1H and 13C NMR data, hydrolysis studies of all complexes by 1H NMR and ESI-MS, photoreaction of 6-TG, photostability of RuTGCl and RuTGBr by 1H NMR. CCDC 879121, 967545 and 967547. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra02960g |
| This journal is © The Royal Society of Chemistry 2014 |