Strong antiferromagnetic interaction in a 3D copper–organic framework and spin-glass-like behaviour in a 1D nickel compound†
Sheng-Yun Liaoa,
Tian-Hao Lia,
Jin-Lei Tiana,
Lin-Yan Yanga,
Wen Gu*ab and
Xin Liu*ab
aDepartment of Chemistry, Nankai University, Tianjin, 300071, China. E-mail: Guwen68@nankai.edu.cn; Liuxin64@nankai.edu.cn
bKey Laboratory of Advanced Energy Material Chemistry, Tianjin, 300071, China
First published on 24th June 2014
Abstract
The in situ hydrothermal reaction of 5-(4-carboxy-1H-1,2,3-triazol-1-yl)isophthalic acid (H3ctia) with M(NO3)2·nH2O (M = Cu, Ni) afforded two new coordination polymers, {[Cu4(tia)4(H2O)3]·H2O}n (1) and {Ni(H2O)6·[Ni2(ctia)2(H2O)6]·2H2O}n (2) (tia2− = 5-(1H-1,2,3-triazol-1-yl)isophthalate). X-ray structural analysis reveals complex 1 is an unusual 3D framework containing D4h paddle-wheel copper units and two kinds of mononuclear copper units, and its resulting structure can be rationalized as a new topology with the Schläfli symbol of {4.82}2{42.85.106.122}{82.10}2{83}2. Compound 2 displays a 1D zigzag chain structure. Strong antiferromagnetic interactions are observed among dinuclear units in complex 1. Interestingly, complex 2 exhibits spin-glass-like behaviour with the spin glass freezing temperature at 15.8 K. In addition, the thermal stability of these compounds was also studied.
Introduction
The construction of coordination networks containing metal ions with magnetic anisotropy is particularly attractive owing to their aesthetic structure and potential application in the field of molecule-based magnetic materials.1 However, the notion of effective design and synthesis of such materials has received less attention. When aiming to achieve such materials, the choice of appropriate bridging ligand and metal ions is of great importance.So far, the majority of magnetic frameworks are first row transition metals (Co, Ni and Cu) complexes.2 That is because they have an adjustable spin quantum number3 and magnetic anisotropy.4
As far as the ligand is concerned, heterocyclic polycarboxylics such as pyridine-2,4-dicarboxylic acid,5 pyrazine-2,3-dicarboxylic acid,6 1H-benzimidazole-5,6-dicarboxylic acid,7 isonicotinic,8 etc. were verified to be candidates due to their versatile coordination conformations and strong coordination ability. It has been demonstrated that the mixed multiple heterobridges constructed by polydentate polyazole9 and conformation-dependent carboxylate groups can efficiently mediate the different magnetic couplings with variable strength and nature.10 H3ctia is a new phenyl heterocyclic polycarboxylic ligand explored by our group and Guo11 (see Scheme 1). We selected phenyl heterocyclic polycarboxylic acid H3ctia (see Scheme 1) as the starting material to assemble different paramagnetic ions to form metal-frameworks in consideration of the following points. (i) Two carboxyl groups and one triazol ring with one carboxyl group lie in meta-position, which may help construct the multi-dimensional structure. (ii) Short bridging such carboxyl and triazol bridging may be responsible for the formation of a metal cluster. (iii) Long bridging across two aromatic rings may block the magnetic interaction among the clusters. Previously, a series of 3D Co–Ln heterometallic, 2D and 1D transition metal coordination polymers based on this ligand were reported.11 Herein, we reported two new complexes, one 3D framework {[Cu4(tia)4(H2O)3]·H2O}n (1) with strong antiferromagnetic interaction and one 1D chain {Ni(H2O)6·[Ni2(ctia)2(H2O)6]·2H2O}n (2) with spin glass behaviour.
Experimental
1 Materials and physical measurements
The reagents and solvents were obtained from commercial sources and used as received. Elemental analyses were determined on a Perkin-Elmer PE 2400 CHNS/O analyzer. IR spectra (KBr pellets) were recorded on a Perkin-Elmer spectrometer in the range 4000–400 cm−1. Temperature- and field-dependent magnetic measurements were carried out on a SQUID-MPMS-XL magnetometer. Diamagnetic corrections were made with Pascal's constants. X-ray powder diffraction (XRPD) intensities were measured on Rigaku D/max-IIIA diffractometer (Cu kα, λ = 1.54056 Å). The single crystalline powder samples were prepared by crushing the crystals and scanned from 3° to 6° with a step of 0.1° s−1. Thermogravimetric analysis were performed on a NETZSCH TG 209 instrument with a heating rate of 10 °C min−1 in the flowing air atmosphere.2 General synthesis procedure
3 X-ray structure determination of complexes 1–2
Diffraction data for complexes 1–2 were collected with a Bruker SMART APEX CCD instrument with graphite monochromatic Mo-Ka radiation (λ = 0.71073 Å). The data were collected at 293(2) K. The absorption corrections were made by multi-scan methods. The structure was solved by charge flipping methods with the program Olex2 and refined by full-matrix least-squares methods on all F2 data with Olex2. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms of water molecules were located in a difference Fourier map and refined isotropically in the final refinement cycles. Other hydrogen atoms were placed in calculated positions and refined by using a riding model. Crystallographic data for 1–2 are given in Table 1. Selected bond lengths and angles are given in Tables S1–S2.†| Identification | Compound 1 | Compound 2 |
|---|---|---|
| Formula | C40H28Cu4N12O20 | C22H36N6Ni3O26 |
| Mwt (g mol−1) | 1250.90 | 976.70 |
| T (K) | 273(2) | 293(2) |
| Crystal system | Triclinic | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 10.3314(15) | 6.958(2) |
| b (Å) | 12.3196(18) | 10.909(4) |
| c (Å) | 19.042(3) | 11.877(4) |
| α (deg) | 106.785(3) | 82.523(10) |
| β (deg) | 93.694(3) | 82.286(10) |
| γ (deg) | 94.284(3) | 71.561(9) |
| Z | 2 | 1 |
| ρ (g cm−3) | 1.803 | 1.922 |
| μ (mm−1) | 1.917 | 1.771 |
| F (000) | 1256 | 502 |
| 2θ scan range (°) | 3.09 to 27.49 | 3.10 to 25.01 |
| Rint | 0.0485 | 0.0482 |
| Reflns collected | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 474 |
5590 |
| Indep reflns | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 233 233 |
2951 |
| Parameters | 691 | 318 |
| R1, ωR2 [I > 2σ(I)] | 0.0679, 0.1774 | 0.0550, 0.1225 |
| R1, ωR2 [all data] | 0.0946, 0.1952 | 0.0701, 0.1298 |
| GOF on F2 | 1.115 | 1.116 |
| Largest residuals (e Å−3) | 1.409, −1.217 | 0.468, −0.568 |
Results and discussion
1 Conversion of H3ctia
Both target compounds were directly isolated by using 5-(4-carboxy-1H-1,2,3-triazol-1-yl)isophthalic acid (H3ctia) as the starting materials. During the course of constructing 1, H3ctia underwent decarboxylation and doubly deprotonation to tia2− (5-(1H-1,2,3-triazol-1-yl)isophthalate) (see Scheme 1). While in compound 2, H3ctia triply deprotonated to ctia3−. Except for the reaction temperature and the pH of the solution, the other reaction conditions are the same, so these two factors may be responsible for the decarboxylation of H3ctia in construction complex 1.2 Structure descriptions
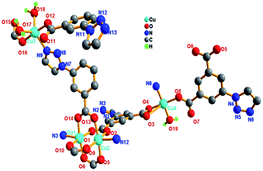
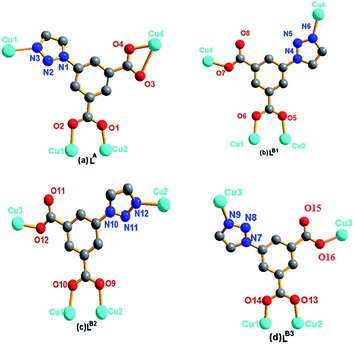 | ||
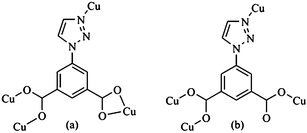
| Fig. 2 Bridging Cu(II) modes of the ligands tia2−. (a) LA: μ4-bridging; (b) LB1: μ4-bridging; (c) LB2: μ4-bridging; (d) LB3: μ4-bridging. | ||
 | ||
| Fig. 3 (a) Paddle-wheel Cu(II) units; (b) Cu3 and Cu4 linked by the ligand tia2− to form 3D framework. (c) Topologically representation for the 3D structure of 1. | ||
Better insight into this framework can be achieved by topology analysis. In 1, the binuclear Cu(II) can be viewed as 6-connected nodes which are connected to two LA, two LB2, one LB1 and one LB3 ligands. While the Cu3, Cu4, the ligands LA and LB can be seen as 3-connected nodes (see Fig. 3(c)). Therefore, this structure can be simplified as an unordinary 4-nodal (3,3,3,6)-connected topological network with the Schläfli symbol of {4.82}2{42.85.106.122}{82.10}2{83}2 {representing the ligand LA and LB2, binuclear Cu(II), LB1 and LB3, mononuclear}.
3 XRPD results
X-ray powder diffraction measurements studies verify the as-obtained bulk substance of complexes 1 and 2 are homogeneous phase (see Fig. S3 in ESI†).4 Magnetic properties
The magnetic properties of 1 and 2 were investigated in the 2.0–300 K range at 1000 Oe. The magnetic susceptibility of 1 versus temperature is shown in Fig. 5. The χMT value is 0.94 cm3 K mol−1 at 300 K, which is significantly smaller than the spin-only value of 1.5 cm3 K mol−1 calculated for four Cu(II) ions (S = 1/2, g = 2). This suggests antiferromagnetic couplings exist between the paramagnetic ions even at room temperature. Upon lowering the temperature, the χMT decreases rapidly to 0.86 cm3 K mol−1 from 300 K to 75 K, then keep constant until 15 K. when temperature continues to decrease, the χMT value slowly increases. The per mole magnetic susceptibility of 1 can viewed as sum of the contributions of one dinuclear Cu(II) unit and two free Cu(II) ions. The magnetic susceptibility of the dinuclear Cu(II) unit can be expressed by the Bleaney–Bowers equation. The χM of 1 is adequately represented by the eqn (1).
 | (1) |
 | ||
| Fig. 5 Temperature dependence of the χMT and χM−1 curve for 1. The solid line represents the best fit. | ||
The least-squares fit to the data (60–300 K) leads to g = 2.17, 2J = −173.4 cm−1, R = ∑(χobsd − χ′cacld)2/∑(χobsd)2 = 3.03 × 10−4. Below 60 K, the data deviate substantially from the dimer model, presumably because of the presence of paramagnetic impurities.
From the view point of the relationship of magnetism and structure, the strong antimagnetic coupling of 1 mainly comes from paddle-wheel binuclear Cu(II) ions16 bridged by tetra-carboxyl groups with short distance of Cu1–Cu2. The drop in χMT to a value of 0.86 cm3 K mol−1 at low temperature, which is very close to 0.75 cm3 K mol−1 for two isolated Cu(II) with S = 1/2, g = 2, indicates the binuclear Cu(II) ions in the paddle-wheel units are strongly antiferromagnetically coupled to each other to a diamagnetic S = 0 ground state. When the temperature drops to 10 K, the χMT begins to increase slightly. That may be ascribed to the result of a minor paramagnetic impurity phase.17
As shown in Fig. 6, complex 2 has a χMT value of 3.67 cm3 K mol−1, which is close to the theoretical value of 3.3 cm3 K mol−1 for S = 1 with g = 2.2.18 Upon cooling from room temperature, the χMT value decrease from 3.67 to 3.48 cm3 K mol−1 at ca. 40 K, which indicates a dominant antiferromagnetic interaction19 between the Ni(II) ions. The magnetic susceptibility above 40 K obeys the Curie–Weiss law with Weiss constant, θ = −4.23 K, and Curie constant, C = 1.141 cm3 K mol−1. Then the χMT value increases rapidly to a maximum of 3.80 cm3 K mol−1 at 26 K and finally drops to 2.05 cm3 K mol−1.
 | ||
| Fig. 6 Temperature dependence of the χMT and χM−1 curve for 2. The solid line represents the Curie Weiss fit. | ||
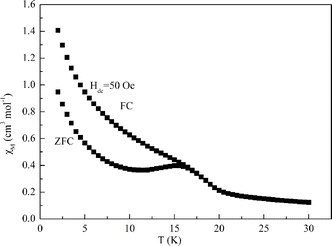
The zero-field alternative-current (ac) magnetic susceptibility was performed under Hac = 3.5 Oe and a frequency of 10–800 Hz (see Fig. S4 in the ESI†). Both of the in-phase and out-of-phase signals, χ′M and χ′′M display a very small frequency-dependent behaviour (see Fig. S4 in the ESI and Fig. S4′†). The shift of the peak temperature (Tp) of χ′M is measured by a parameter φ = (ΔTp/Tp)/Δ(log f) = 0.01, which is in the range of a spin-glass.21 Unfortunately, the peaks of the out-of-phase are too small and close to the noise of the instrument, so the relaxation time (τ) and Δ/kB can't be calculated from the Arrhenius law.22
To further investigate the magnetic behaviour of 2 at low temperature, the field-cooled (FC) and zero-field-cooled (ZFC) magnetization measurements were performed at 50 Oe in the 2–30 K range (see Fig. 7). The temperature correspondent to the ZFC maximum (15.8 K) is the spin glass freezing temperature.20
In addition, the field-dependent isothermal magnetization M(H) was performed at 2 K, a value of 4.8 NμB at 40 kOe is far below the saturation value of 6.6 NμB expected for the Ni3 unit because of the spin glass component (see Fig. S5 in the ESI†). Magnetization curve versus applied field measured at 2.0 K of complex 2 did not exhibit an obvious hysteresis effect under our experimental conditions (Fig. S5 in the ESI: inset†). Thus, it is difficult for us to further confirm the concrete magnetic behavior of 2. But we can confirm complex 2 should be a spin glass.
5 Thermal stability properties
The TGA curve of 2 exhibits an initial weight loss from 100 °C to 300 °C, with the observed weight loss of 25.3% corresponding to the release of lattice and coordinated-water molecules (calcd 25.8%) (see Fig. 8). After the water molecules were released, the skeleton collapse begins at about 300 °C, which is lower than those of coordinated polymers reported previously.23 That may be because complex 2 contains too much water molecules. The TGA diagram of complex 1 displays the loss of lattice water molecules at 170 °C, which is higher than complex 2. This can be ascribed to the fact that the three dimension framework can hinder the escape of the water molecules in some degree. Above 500 °C, there is almost no weight loss in complexes 1 and 2. The remnants of complexes 1 and 2 are 23.8% and 26.5%, respectively.Conclusions
In this contribution, we have presented two new metal–organic frameworks with the starting materials H3ctia. Complex 1 is a 3D metal–organic framework based on the D4h paddle-wheel units [Cu2(O2CR)4N2] which were connected by two kinds five coordinated mononuclear Cu(II) units as linkers. Among the paddle-wheel units, the strong antiferromagnetic interaction can be observed between the Cu atoms. Compound 2 displays 1D zigzag chain structure. Interestingly, complex 2 exhibits spin-glass-behaviour with the spin glass freezing temperature at 15.8 K. Furthermore, the thermal stability of these compounds show the framework structure can affect the release of lattice water molecules.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 22371103 and no. 20771062) and the Tianjin Natural Science Foundation (no. 08JCZDJC21100).Notes and references
- (a) J. W. Yoo, C. Y. Chen, H. W. Jang, C. W. Bark, V. N. Prigodin, C. B. Em and A. J. Epstein, Nat. Mater., 2010, 9, 638 CrossRef CAS PubMed; (b) W. R. Entley and G. S. Girolami, Science, 1995, 268, 397 CAS; (c) J. S. Miller, Angew. Chem., Int. Ed., 2003, 42, 47 Search PubMed; (d) X. He and D. Antonelli, Angew. Chem., Int. Ed., 2002, 41, 214 CrossRef CAS; (e) L. Bogani, L. Cavigli, M. Gurioli, R. L. Novak, M. Mannini, A. Caneschi, F. Pineider, R. Sessoli, M. Clemente-león, E. Coronado, A. Cornia and D. Gatteschi, Adv. Mater., 2007, 19, 3906 CrossRef CAS; (f) J. S. Miller and M. Drillon, ChemPhysChem, 2002, 3, 380 Search PubMed; (g) M. Lopez, H. H. Zhao, A. V. Prosvirin, A. Chouai, M. Shatruk and K. R. Dumbar, Chem. Commun., 2007, 4611 RSC; (h) M. Mito, K. Iriuchi, H. Deguchi and T. I. Kishine, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 012406 CrossRef; (i) R. Garde, F. Villain and M. Verdaguer, J. Am. Chem. Soc., 2002, 124, 1053 CrossRef PubMed; (j) S. I. Ohkoshi and K. Hashimoto, J. Am. Chem. Soc., 1999, 121, 1059 CrossRef.
- (a) R. C. Poulten, M. J. Page, A. G. Algarra, J. J. Le Roy, I. López, E. Carter, A. Llobet, S. A. Macgregor, M. F. Mahon and D. M. Murphy, J. Am. Chem. Soc., 2013, 135, 13640–13643 CrossRef CAS PubMed; (b) R. J. Kuppler, D. J. Timmons, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D. Young, D. Yuan, D. Zhao, W. Zhuang and H.-C. Zhou, Coord. Chem. Rev., 2009, 253, 3042–3066 CrossRef CAS PubMed; (c) W. Ouellette, A. V. Prosvirin, K. Whitenack, K. R. Dunbar and J. Zubieta, Angew. Chem., Int. Ed., 2009, 48, 2140–2143 CrossRef CAS PubMed; (d) P. Jensen, S. R. Batten, B. Moubaraki and K. S. Murray, Chem. Commun., 2000, 793–794 RSC.
- (a) Y.-Q. Lan, S.-L. Li, X.-L. Wang, K.-Z. Shao, D.-Y. Du, H.-Y. Zang and Z.-M. Su, Inorg. Chem., 2008, 47, 8179–8187 CrossRef CAS PubMed; (b) L. R. MacGillivray, S. Subramanian and M. J. Zaworotko, J. Am. Chem. Soc., 1994, 1325–1326 CAS; (c) R. Schenker, B. S. Mandimutsira, C. G. Riordan and T. C. Brunold, J. Am. Chem. Soc., 2002, 124, 13842–13855 CrossRef CAS PubMed; (d) B. M. Weckhuysen, A. A. Verberckmoes, M. G. Uytterhoeven, F. E. Mabbs, D. Collison, E. de Boer and R. A. Schoonheydt, J. Phys. Chem. B, 2000, 104, 37–42 CrossRef CAS; (e) S. Yamaguchi, Y. Okimoto, H. Taniguchi and Y. Tokura, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, 2926–2935 CrossRef.
- (a) T. D. Harris, M. V. Bennett, R. Clerac and J. R. Long, J. Am. Chem. Soc., 2010, 132, 3980–3988 CrossRef CAS PubMed; (b) J. H. Lim, J. H. Yoon, H. C. Kim and C. S. Hong, Angew. Chem., Int. Ed., 2006, 45, 7424–7426 CrossRef CAS PubMed; (c) J. L. Manson, J. Gu, J. A. Schlueter and H.-H. Wang, Inorg. Chem., 2003, 42, 3950–3955 CrossRef CAS PubMed; (d) M. A. Lawandy, X. Huang, R.-J. Wang, J. Li, J. Y. Lu, T. Yuen and C. Lin, Inorg. Chem., 1999, 38, 5410–5414 CrossRef CAS.
- (a) M.-S. Liu, Q.-Y. Yu, Y.-P. Cai, C.-Y. Su, X.-M. Lin, X.-X. Zhou and J.-W. Cai, Cryst. Growth Des., 2008, 8, 4083–4091 CrossRef CAS; (b) X. Wang, C. Qin, E. Wang, Y. Li, N. Hao, C. Hu and L. Xu, Inorg. Chem., 2004, 43, 1850–1856 CrossRef CAS PubMed.
- (a) L.-L. Wen, Z.-D. Lu, X.-M. Ren, C.-Y. Duan, Q.-J. Meng and S. Gao, Cryst. Growth Des., 2008, 9, 227–238 CrossRef; (b) F.-Q. Wang, W.-H. Mu, X.-J. Zheng, L.-C. Li, D.-C. Fang and L.-P. Jin, Inorg. Chem., 2008, 47, 5225–5233 CrossRef CAS PubMed.
- (a) F. Stomeo, C. Lincheneau, J. P. Leonard, J. E. O'Brien, R. D. Peacock, C. P. McCoy and T. Gunnlaugsson, J. Am. Chem. Soc., 2009, 131, 9636–9637 CrossRef CAS PubMed; (b) Y. Wei, Y. Yu and K. Wu, Cryst. Growth Des., 2008, 8, 2087–2089 CrossRef CAS.
- (a) R. Sekiya, S.-i. Nishikiori and K. Ogura, J. Am. Chem. Soc., 2004, 126, 16587–16600 CrossRef CAS PubMed; (b) R. Sekiya and S. i. Nishikiori, Chem.–Eur. J., 2002, 8, 4803–4810 CrossRef CAS.
- (a) E.-C. Yang, Z.-Y. Liu, T.-Y. Liu, L.-L. Li and X.-J. Zhao, Dalton Trans., 2011, 40, 8132–8139 RSC; (b) Z.-G. Gu, Y.-F. Xu, X.-H. Zhou, J.-L. Zuo and X.-Z. You, Cryst. Growth Des., 2008, 8, 1306–1312 CrossRef CAS; (c) R. Bronisz, Inorg. Chem., 2007, 46, 6733–6739 CrossRef CAS PubMed.
- (a) Y. F. Zeng, X. Hu, F. C. Liu and X. H. Bu, Chem. Soc. Rev., 2009, 38, 469 RSC; (b) J. M. Rueff, N. Masciocchi, P. Rabu, A. Sironi and A. Skoulios, Chem.–Eur. J., 2002, 8, 1813 CrossRef CAS.
- (a) S. Y. Liao, W. Gu, L. Y. Yang, T. H. Li, J. L. Tian, Li. Wang, M. Zhang and X. Liu, Cryst. Growth Des., 2012, 12, 3927 CrossRef CAS; (b) S. Y. Liao, W. Gu, L. Y. Yang, T. H. Li, Li. Wang, M. Zhang and X. Liu, Polyhedron, 2012, 36, 38–44 CrossRef CAS PubMed; (c) Y. Li, W. Q. Zou, M. F. Wu, J. D. Lin, F. K. Zheng, Z. F. Liu, S. H. Wang, G. C. Guo and J. S. Huang, CrystEngComm, 2011, 13, 3868 RSC.
- (a) P. Pierrat, S. Vanderheiden, T. Muller and S. Bräse, Chem. Commun., 2009, 1748 RSC; (b) C. Camp, S. Dorbes, C. Picard and E. Benoist, Tetrahedron Lett., 2008, 49, 1979–1983 CrossRef CAS PubMed.
- (a) Y. H. Shi, W. Z. Chen, K. D. John, R. E. D. Re, J. L. Cohn, G. L. Xu, J. L. Eglin, A. P. Sattelberger, C. R. Hare and T. Ren, Inorg. Chem., 2005, 44, 5719 CrossRef CAS PubMed; (b) B. S. Kennon, J. H. Her, P. W. Stephens and J. S. Miller, Inorg. Chem., 2009, 48, 6117 CrossRef CAS PubMed; (c) M. H. Chisholm, K. C. Glasgow, L. J. Klein, A. M. Macintosh and D. G. Peters, Inorg. Chem., 2000, 39, 4354 CrossRef CAS; (d) F. A. Cotton, D. S. Martin, T. R. Webb and T. J. Peters, Inorg. Chem., 1976, 15, 1199 CrossRef CAS; (e) D. S. Martin, R. A. Newman and P. E. Fanwick, Inorg. Chem., 1979, 18, 251 Search PubMed.
- (a) M. V. Marinho, M. I. Yoshida, K. J. Guedes, K. Krambrock, A. J. Bortoluzzi, M. Hömer, F. C. Machado and W. M. Teles, Inorg. Chem., 2004, 43, 1539 CrossRef CAS PubMed; (b) S. Q. Su, Z. Y. Guo, G. H. Li, R. P. Deng, S. Y. Song, C. Qin, C. L. Pan, H. Guo, F. Cao, S. Wang and H. J. Zhang, Dalton Trans., 2010, 39, 3123 RSC.
- Y. Li, W. Q. Zou, M. F. Wu, J. D. Lin, F. K. Zheng, Z. F. Liu, S. H. Wang, G. C. Guo and J. S. Huang, CrystEngComm, 2011, 13, 3868 RSC.
- (a) L. Gutiérrez, G. Alzuet, J. Borrás, A. Castiñeiras, A. Rodríguez-Fortea and E. Ruiz, Inorg. Chem., 2001, 40, 3089 CrossRef PubMed; (b) J. S. Valentine, A. J. Silverstein and Z. G. Soos, J. Am. Chem. Soc., 1974, 96, 97 CrossRef CAS.
- J. Garcia-Ruiz, J. Blasco, F. Hueso-Urena and M. Moreno-Carretero, J. Mater. Sci., 1998, 33, 2103–2109 CrossRef CAS.
- D. K. Gao, Y. Z. Li and L. M. Zheng, Inorg. Chem., 2007, 46, 7571 CrossRef PubMed.
- Y. Yafet and C. Kittel, Phys. Rev., 1952, 87, 290 CrossRef CAS.
- (a) Y. G. Huang, D. Q. Yuan, L. Pan, F. L. Jiang, M. Y. Wu, X. D. Zhang, W. Wei, Q. Gao, J. Y. Lee and M. C. Hong, Inorg. Chem., 2007, 46, 9609 CrossRef CAS PubMed; (b) M. R. Plout, S. Vilminot, M. Guillot and M. Kurmoo, Chem. Mater., 2002, 14, 3829 CrossRef.
- (a) W. E. Buschmannn, J. Ensling, P. Gütlich and J. S. Miller, Chem.–Eur. J., 1999, 5, 3019 CrossRef; (b) M. Arai, M. Miyake and M. Yamada, J. Phys. Chem. C, 2008, 112, 1953 CrossRef CAS; (c) G. Layrac, D. Tichit, J. Larionova, Y. Guari and C. Guérin, J. Phys. Chem. C, 2011, 115, 3263 CrossRef CAS; (d) J. Lefebvre, P. Tyagi, S. Trudel, V. Pacradouni, C. Kaiser, J. E. Sonier and D. B. Leznoff, Inorg. Chem., 2009, 48, 55 CrossRef CAS PubMed.
- (a) D. G. Branzea, L. Sorace, C. Maxim, M. Andruh and A. Caneschi, Inorg. Chem., 2008, 47, 6590 CrossRef CAS PubMed; (b) K. C. Mondal, G. E. Kostakis, Y. H. Lan, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 9205 CrossRef CAS PubMed.
- (a) D. Moon, J. Song, B. J. Kim, B. J. Suh and M. S. Lah, Inorg. Chem., 2004, 43, 8230 CrossRef CAS PubMed; (b) X. J. Gu and D. F. Xue, Cryst. Growth Des., 2007, 9, 1727 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Selected bonds and angles for 1 and 2, powder X-ray diffraction patterns of 1 and 2, Temperature dependence of ac susceptibility at various frequencies of 2 and Field dependence of magnetization and the hysteresis loop at 2 K for 2. CCDC 901576 and 901577. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra02942a |
| This journal is © The Royal Society of Chemistry 2014 |