Adsorption, photodegradation and antibacterial study of graphene–Fe3O4 nanocomposite for multipurpose water purification application†
Abstract
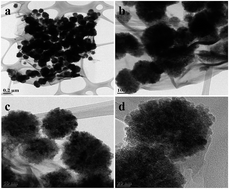
Graphene–Fe3O4 (G–Fe3O4) composite was prepared from graphene oxide (GO) and FeCl3·6H2O by a one-step solvothermal route. The as-prepared composite was characterized by field-emission scanning electron microscopy, transmission electron microscopy, dynamic light scattering and X-ray powder diffraction. SEM analysis shows the presence of Fe3O4 spheres with size ranging between 200 and 250 nm, which are distributed and firmly anchored onto the wrinkled graphene layers with a high density. The resulting G–Fe3O4 composite shows extraordinary adsorption capacity and fast adsorption rates for the removal of Pb metal ions and organic dyes from aqueous solution. The adsorption isotherm and thermodynamics were investigated in detail, and the results show that the adsorption data was best fitted with the Langmuir adsorption isotherm model. From the thermodynamics investigation, it was found that the adsorption process is spontaneous and endothermic in nature. Thus, the as-prepared composite can be effectively utilized for the removal of various heavy metal ions and organic dyes. Simultaneously, the photodegradation of methylene blue was studied, and the recycling degradation capacity of dye by G–Fe3O4 was analyzed up to 5 cycles, which remained consistent up to ∼97% degradation of the methylene blue dye. Although iron oxide has an affinity towards bacterial cells, its composite with graphene still show antibacterial property. Almost 99.56% cells were viable when treated with Fe3O4 nanoparticle, whereas with the composite barely 3% cells survived. Later, the release of ROS was also investigated by membrane and oxidative stress assay. Total protein degradation was analyzed to confirm the effect of the G–Fe3O4 composite on E. coli cells.

 Please wait while we load your content...
Please wait while we load your content...