Vacuum processable donor material based on dithieno[3,2-b:2′,3′-d]thiophene and pyrene for efficient organic solar cells†
Jongchul Kwon‡
a,
Tae-Min Kim‡b,
Hong-Se Oha,
Jang-Joo Kim*b and
Jong-In Hong*a
aDepartment of Chemistry, Seoul National University, Seoul 151-747, Korea. E-mail: jihong@snu.ac.kr; Fax: +82-2-889-1568; Tel: +82-2-874-2456 Tel: +82-2-880-6682
bDepartment of Materials Science and Engineering and the Center for Organic Light-Emitting Diode, Seoul National University, Seoul 151-742, Korea. E-mail: jjkim@snu.ac.kr
First published on 23rd May 2014
Abstract
We have developed a new donor material, 2,6-di(pyren-1-yl) dithieno[3,2-b:2′,3′-d]thiophene (DDT), consisting of dithieno[3,2-b:2′,3′-d]thiophene (DTT) and pyrene units. The bulk heterojunction (BHJ) device based on DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 exhibited an efficient power conversion efficiency (PCE) of 3.60%, under simulated AM 1.5 solar irradiation at 100 mW cm−2.
4 exhibited an efficient power conversion efficiency (PCE) of 3.60%, under simulated AM 1.5 solar irradiation at 100 mW cm−2.
Organic solar cells (OSCs) are emerging as a clean and renewable energy resource due to their unique features including low-cost manufacturing, easy synthesis of active layer materials, possibility of flexible panels, easy device fabrication, and other potential applications.1
Since the first report of OSCs by Tang,1 researchers have focused on improving the power conversion efficiency (PCE) of the devices using new organic small molecules. In recent years, the PCE of OSCs has been steadily improved by the use of various organic small molecules.2–4 Further, the PCEs of 8–9% have been achieved for polymer and small molecule based OSCs with the most promising bulk heterojunction (BHJ) architecture.5
Pyrene-based organic materials have been utilized as active materials of organic thin-film transistors (OTFTs), and blue light-emitting and electron-transporting materials in organic light-emitting diodes (OLEDs), and donor materials for OSCs.6–10 Oligothiophene-based organic materials have been widely investigated for optoelectronic applications such as OSCs, OTFTs, and OLEDs, owing to their light-emitting and absorbing, hole-transporting, and charge carrying transport properties.11 In particular, linearly fused oligothiophene-based compounds derived from dithieno[3,2-b:2′,3′-d]thiophene (DTT) have been utilized in a variety of organic optoelectronic applications, because the DDT unit has a relatively high hole mobility due to the fused thiophene core.12–15 Therefore, the synthesis of DDT derivatives has received much attention.16 However, to the best of our knowledge, DTT-based small molecules have not been successfully utilized for high performance OSCs.17
In this paper, we report on the development of a thermally stable and efficient donor material, 2,6-di(pyren-1-yl)dithieno[3,2-b:2′,3′-d]thiophene (DDT), consisting of DTT and pyrene units, for OSCs. The device performance was optimized at DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; the maximum open circuit voltage (Voc), short circuit current (Jsc), and PCE value of the device were 0.98 V, 9.24 mA cm−2, and 3.60%, respectively, under simulated AM 1.5 solar irradiation at 100 mW cm−2.
4; the maximum open circuit voltage (Voc), short circuit current (Jsc), and PCE value of the device were 0.98 V, 9.24 mA cm−2, and 3.60%, respectively, under simulated AM 1.5 solar irradiation at 100 mW cm−2.
Compound DDT was synthesized by the palladium catalyzed Suzuki cross coupling reaction between 2,6-dibromodithieno[3,2-b:2′,3′-d]thiophene18 and 1-pyreneboronic acid (Scheme 1). Compound DDT was identified by HR-mass and EA analyses.
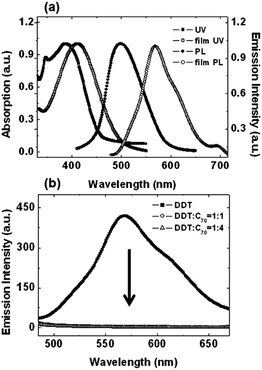
Fig. 1a shows the absorption (UV) and emission (PL) spectra of DDT in CH2Cl2 and in solid film. The UV spectrum of DDT in CH2Cl2 shows a main absorption band with λmax at 387 nm, which originates from the DTT and pyrene unit. The UV spectrum of DDT in solid film shows an absorption maximum band at 412 nm, which is 25 nm red-shifted when compared to the absorption in CH2Cl2. The PL spectrum of DDT shows maximum bands at 499 nm in CH2Cl2 and at 569 nm in film, which is 70 nm red-shifted when compared to the absorption in CH2Cl2. The UV and PL spectra of the solid film of DDT were both red-shifted relative to the CH2Cl2 solution spectra, presumably owing to the more planar structure of DDT in the film than in CH2Cl2. Fig. 1b and S1† show the PL spectra of DDT in neat film and the blended film of DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The PL spectra of DDT in DDT
4. The PL spectra of DDT in DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 blended film were significantly quenched by C70. That means that an efficient intermolecular photoinduced charge transfer (PICT) process from the excited state of DDT to C70 occurred. This PICT process of DDT is similar to the case of conducting conjugated polymer and fullerene blend system.3g
4 blended film were significantly quenched by C70. That means that an efficient intermolecular photoinduced charge transfer (PICT) process from the excited state of DDT to C70 occurred. This PICT process of DDT is similar to the case of conducting conjugated polymer and fullerene blend system.3g
 | ||
| Fig. 1 UV spectra (a) and PL spectra (a and b) of DDT at 400 nm excitation in 0.02 mM CH2Cl2 and in solid film. | ||
The thermal stability of DDT was investigated by thermo-gravimetric analysis (TGA) and differential scanning calorimetry (DSC). DDT shows a high thermal stability of Td = 432 °C, as revealed by the TGA thermogram (Fig. 2), suggesting that DDT is stable during the vacuum thermal sublimation process used in OSC fabrication. When the sample of DDT was heated, a melting peak was observed at around 358 °C (Fig. S2†).
As shown in Fig. S3†, the cyclic voltammograms (CVs) of DDT shows irreversible oxidation process in 1,2-dichlorobenzene (ODCB). The oxidation potential energy of DDT is observed at Eonset = 1.00 V, calibrated against the ferrocene/ferrocenium (Fc/Fc+) redox couple. The highest occupied molecular orbital (HOMO) energy level of DDT (−5.66 eV) is calculated from Eonset after correction against the Fc/Fc+ redox couple. The lowest unoccupied molecular orbital (LUMO) energy level of DDT (−2.95 eV) is calculated from the CV and cross sectional wavelength between the UV and PL spectra (Table 1).19
| UV (nm) | PL (nm) | HOMO (eV) | LUMO (eV) | Td (°C) | Tm (°C) | |||
|---|---|---|---|---|---|---|---|---|
| sol | film | sol | film | |||||
| DDT | 387 | 412 | 499 | 569 | 5.66 | 2.95 | 432 | 358 |
DDT-deposited films exhibit no diffraction peaks present in the X-ray diffraction (XRD) patterns, indicating that the DDT molecule has poor crystalline morphology in the solid films (Fig. S4, ESI†).
To investigate the correlation between the molecular structure and the photophysical properties, we performed molecular orbital calculations using density functional theory (DFT). The ground state geometries of DDT were optimized in vacuum using the B3LYP/6-31 G* level in the Gaussian03 package. The optimized structures and the frontier molecular orbitals are shown in Fig. 3. The HOMO level of DDT is located on both the DTT and pyrene units. The LUMO level of DDT is mainly located on the pyrene unit.
To investigate the photovoltaic properties of DDT, we fabricated planar heterojunction (PHJ) devices with a configuration of ITO/MoO3 (5 nm)/DDT (10, 20, 30 nm)/C70 (40 nm)/2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP) (8 nm)/Al (100 nm). Fig. S5, ESI† shows the current density (J) vs. voltage (V) characteristic curves of the PHJ solar cells. All the PHJ devices exhibit the poor photovoltaic behavior. The optimized 20 nm DDT-based device exhibited a Jsc of 3.15 mA cm−2, a Voc of 0.76 V, a fill factor (FF) of 0.22; thus a PCE of 0.52% was obtained. In contrast, the device with a 30 nm DDT based device had a reduced Jsc of 0.71 mA cm−2, a Voc of 0.88 V, and a FF of 0.17; thus, a reduced PCE of 0.11%. The low-lying HOMO energy levels of DDT results in a large energy difference relative to the LUMO of fullerenes, leading to the relatively large Voc values. The Voc value increases from 0.60 V to 0.88 V with increasing the thickness of DDT film from 10 nm to 30 nm.20 Overall, the PCE of DDT-based PHJ devices were not high compared with other small-molecule-based OSCs due to the short absorption range and poor crystalline property of DDT (Fig. S4, ESI†). Fig. S5, ESI† shows the incident photon to current conversion efficiency (IPCE) spectra of PHJ solar cells. PHJ solar cells show IPCE values of 30% at 526 nm for 20 nm DDT based device and 25% at 538 nm for 30 nm DDT based device (Fig. S5, ESI†).
To improve the PCE, we fabricated bulk heterojunction devices with a configuration of ITO/MoO3 (5 nm)/DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (50 nm)/BCP (8 nm)/Al (100 nm). Fig. 4a shows the J–V characteristic curves of the BHJ solar cells. The DDT
4 (50 nm)/BCP (8 nm)/Al (100 nm). Fig. 4a shows the J–V characteristic curves of the BHJ solar cells. The DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based device exhibited a Jsc of 2.27 mA cm−2, a Voc of 0.91 V, and a PCE of 0.38%. On the other hand, the DDT
1 based device exhibited a Jsc of 2.27 mA cm−2, a Voc of 0.91 V, and a PCE of 0.38%. On the other hand, the DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 based device exhibited a significantly increased efficiency: a Jsc of 9.24 mA cm−2 a Voc of 0.98 V, and a PCE of 3.60% (Fig. 4a). The higher PCE of the DDT
4 based device exhibited a significantly increased efficiency: a Jsc of 9.24 mA cm−2 a Voc of 0.98 V, and a PCE of 3.60% (Fig. 4a). The higher PCE of the DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 based device can be explained by the increased dissociation of excitons (Fig. S1, ESI†) and increased carrier mobility at a low donor concentration.21
4 based device can be explained by the increased dissociation of excitons (Fig. S1, ESI†) and increased carrier mobility at a low donor concentration.21
 | ||
Fig. 4 (a) J–V characteristic curves and (b) IPCE spectra of ITO/MoO3 (5 nm)/DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1 C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,1 1,1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (50 nm)/BCP(8 nm)/Al (100 nm) device under simulated AM 1.5 solar irradiation at 100 mW cm−2. 4 (50 nm)/BCP(8 nm)/Al (100 nm) device under simulated AM 1.5 solar irradiation at 100 mW cm−2. | ||
The dependence of the photocurrent (Jph) on the light intensity (I), taken from J–V characteristic curves of the device with DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and DDT
1 and DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Fig. S6, ESI†), reveals which recombination process is dominant (Fig. 5). The light intensity-dependent photocurrent shows a power law behavior and α is taken from the slope in the log-scale. The devices with DDT
4 (Fig. S6, ESI†), reveals which recombination process is dominant (Fig. 5). The light intensity-dependent photocurrent shows a power law behavior and α is taken from the slope in the log-scale. The devices with DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and DDT
1 and DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 show values of α = 0.54 and α = 0.82, respectively. The square-root dependence of the photocurrent on the light intensity in the device with DDT
4 show values of α = 0.54 and α = 0.82, respectively. The square-root dependence of the photocurrent on the light intensity in the device with DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 suggests that the bimolecular recombination process is dominant.22 In other words, it can be explained that the path for the carrier transport is more or less prevented because of the mixing of the donor and the acceptor molecules. On the contrary, the device with DDT
1 suggests that the bimolecular recombination process is dominant.22 In other words, it can be explained that the path for the carrier transport is more or less prevented because of the mixing of the donor and the acceptor molecules. On the contrary, the device with DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 shows the three-fourth power law dependence of the photocurrent on the light intensity, which indicates that the space-charge limited recombination process is dominant. This means that the path for the electron and the hole transport is relatively well built in the active layer.22 It is noteworthy that the PCE of 3.60%, to the best of our knowledge, is the highest value among DTT-based OSC devices due to the efficient exciton dissociation and space charge limited recombination process. Fig. 4b shows the IPCE spectra of BHJ solar cells utilizing DDT and C70. The IPCE spectra of the BHJ devices based on DDT and C70 show a broad response extending from 300 to 700 nm. BHJ devices based on DDT
4 shows the three-fourth power law dependence of the photocurrent on the light intensity, which indicates that the space-charge limited recombination process is dominant. This means that the path for the electron and the hole transport is relatively well built in the active layer.22 It is noteworthy that the PCE of 3.60%, to the best of our knowledge, is the highest value among DTT-based OSC devices due to the efficient exciton dissociation and space charge limited recombination process. Fig. 4b shows the IPCE spectra of BHJ solar cells utilizing DDT and C70. The IPCE spectra of the BHJ devices based on DDT and C70 show a broad response extending from 300 to 700 nm. BHJ devices based on DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 show IPCE values with a maximum of 41% at 394 nm for DDT
C70 show IPCE values with a maximum of 41% at 394 nm for DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based device and 60% at 512 nm for DDT
1 based device and 60% at 512 nm for DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 based device (Fig. 4b). The higher PCE and Jsc for the device based on DDT
4 based device (Fig. 4b). The higher PCE and Jsc for the device based on DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 compared to the device based on DDT
4 compared to the device based on DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is reflected in higher IPCE values in the long wavelength region. The overall device performance data of DDT are summarized in Table 2.
1 is reflected in higher IPCE values in the long wavelength region. The overall device performance data of DDT are summarized in Table 2.
| Material | Voc (V) | Jsc (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|
| DDT, 10 nm | 0.60 | 1.02 | 0.16 | 0.10 |
| DDT, 20 nm | 0.76 | 3.15 | 0.22 | 0.52 |
| DDT, 30 nm | 0.88 | 0.71 | 0.17 | 0.11 |
DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1 C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.91 | 2.27 | 0.18 | 0.38 |
DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1 C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0.98 | 9.24 | 0.40 | 3.60 |
In order to explain the device performance difference when using different ratios of DDT and C70, we investigated the morphology of these DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 blended films by atomic force microscopy (AFM).23 The AFM images of the blended film of DDT
C70 blended films by atomic force microscopy (AFM).23 The AFM images of the blended film of DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 are shown in Fig. 6. The morphology of DDT film exhibits the formation of an aggregated large domain (Fig. S7, ESI†). The morphology of DDT
C70 are shown in Fig. 6. The morphology of DDT film exhibits the formation of an aggregated large domain (Fig. S7, ESI†). The morphology of DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blended film exhibits the formation of an aggregated large domain (Fig. 6a). These aggregated domains of DDT and DDT
1 blended film exhibits the formation of an aggregated large domain (Fig. 6a). These aggregated domains of DDT and DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blended films might hinder the efficient exciton dissociation and carrier transport, resulting in low Jsc and PCE. On the other hand, the morphology of DDT
1 blended films might hinder the efficient exciton dissociation and carrier transport, resulting in low Jsc and PCE. On the other hand, the morphology of DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 blended film exhibits the formation of a relatively small domain (Fig. 6b), which presumably leads to a higher Jsc and a high PCE of 3.60%.
4 blended film exhibits the formation of a relatively small domain (Fig. 6b), which presumably leads to a higher Jsc and a high PCE of 3.60%.
Conclusions
We have demonstrated thermally stable and efficient donor material DDT, consisting of DDT and pyrene units. The maximum Voc, Jsc and PCE values of the BHJ device based on DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were 0.98 V, 9.24 mA cm−2, and 3.60%, respectively, under simulated AM 1.5 solar irradiation at 100 mW cm−2. A PCE of 3.60%, to the best of our knowledge, is the highest efficiency among OSC devices fabricated using DTT derivatives.
4 were 0.98 V, 9.24 mA cm−2, and 3.60%, respectively, under simulated AM 1.5 solar irradiation at 100 mW cm−2. A PCE of 3.60%, to the best of our knowledge, is the highest efficiency among OSC devices fabricated using DTT derivatives.
Acknowledgements
This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) of Korea for the Center for Next Generation Dye-sensitized Solar Cells (no. 2008-0061903), and by the New & Renewable Energy Technology Development Program of the KETEP grant (no. 20113020010070) funded by the Ministry of Knowledge Economy.References
- C. W. Tang, App. Phys. Lett., 1986, 48, 183 CrossRef CAS PubMed.
- A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020 CrossRef CAS PubMed.
- (a) B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef CAS PubMed; (b) Y. Shirota, J. Mater. Chem., 2000, 10, 1 RSC; (c) Y. Shirota, J. Mater. Chem., 2005, 15, 75 RSC; (d) J. Roncali, Acc. Chem. Res., 2009, 42, 1719 CrossRef CAS PubMed; (e) D. Demeter, T. Rousseau and J. Roncali, RSC Adv., 2013, 3, 704 RSC; (f) V. Jeux, D. Demeter, P. Leriche and J. Roncali, RSC Adv., 2013, 3, 5811 RSC; (g) N. S. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474 CAS.
- (a) J. Kwon, W. Lee, J.-y. Kim, S. Noh, C. Lee and J.-I. Hong, New J. Chem., 2010, 34, 744 RSC; (b) J. Kwon, M. K. Kim, J.-P. Hong, W. Lee, S. Noh, C. Lee, S. Lee and J.-I. Hong, Org. Electron., 2010, 11, 1288 CrossRef CAS PubMed; (c) J. Kwon, J.-P. Hong, W. Lee, S. Noh, C. Lee, S. Lee and J.-I. Hong, Org. Electron., 2010, 11, 1103 CrossRef CAS PubMed; (d) J. Kwon, M. K. Kim, J.-P. Hong, W. Lee, S. Lee and J.-I. Hong, Bull. Korean Chem. Soc., 2013, 34, 1355 CrossRef CAS.
- (a) Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 593 CrossRef CAS; (b) J. Zhou, Y. Zuo, X. Wan, G. Long, Q. Zhang, W. Ni, Y. Liu, Z. Li, G. He, C. Li, B. Kan, M. Li and Y. Chen, J. Am. Chem. Soc., 2013, 135, 8484 CrossRef CAS PubMed.
- F. Moggia, C. Videlot-Ackermann, J. Ackermann, P. Raynal, H. Brisset and F. Fages, J. Mater. Chem., 2006, 16, 2380 RSC.
- (a) K.-C. Wu, P.-J. Ku, C.-S. Lin, H.-T. Shih, F.-I. Wu, M.-J. Huang, J.-J. Lin, I.-C. Chen and C.-H. Cheng, Adv. Funct. Mater., 2008, 18, 67 CrossRef CAS; (b) J. Kwon, J.-P. Hong, S. Lee and J.-I. Hong, New J. Chem., 2013, 37, 2881 RSC.
- H.-Y. Oh, C. Lee and S. Lee, Org. Electron., 2009, 10, 163 CrossRef CAS PubMed.
- T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260 CrossRef CAS PubMed.
- (a) J. Kwon, J.-P. Hong, S. Noh, T.-M. Kim, J.-J. Kim, C. Lee, S. Lee and J.-I. Hong, New J. Chem., 2012, 36, 1813 RSC; (b) J.-W. Mun, I. Cho, D. Lee, W. S. Yoon, O. K. Kwon, C. Lee and S. Y. Park, Org. Electron., 2013, 14, 2341 CrossRef CAS PubMed; (c) O. P. Lee, A. T. Yiu, P. M. Beaujuge, C. H. Woo, T. W. Holcombe, J. E. Millstone, J. D. Douglas, M. S. Chen and J. M. J. Fréchet, Adv. Funct. Mater., 2011, 23, 5359 CrossRef CAS PubMed.
- (a) J. E. Anthony, Chem. Rev., 2006, 106, 5028 CrossRef CAS PubMed; (b) J. E. Anthony, Angew. Chem., Int. Ed., 2008, 47, 452 CrossRef CAS PubMed.
- X. C. Li, H. Sirringhaus, F. Garnier, A. B. Holmes, S. C. Moratti, N. Feeder, W. Clegg, S. J. Teat and R. H. Friend, J. Am. Chem. Soc., 1998, 120, 2206 CrossRef CAS.
- L. Wang, Q. Chen, G. B. Pan, L. J. Wan, S. M. Zhang, X. W. Zhan, B. H. Northrop and P. J. Stang, J. Am. Chem. Soc., 2008, 130, 13433 CrossRef CAS PubMed.
- L. Zhang, L. Tan, Z. H. Wang, W. P. Hu and D. B. Zhu, Chem. Mater., 2009, 21, 1993 CrossRef CAS.
- X. W. Zhan, Z. A. Tan, E. J. Zhou, Y. F. Li, R. Misra, A. Grant, B. Domercq, X. H. Zhang, Z. S. An, X. Zhang, S. Barlow, B. Kippelen and S. R. Marder, J. Mater. Chem., 2009, 19, 5794 RSC.
- J. Frey, A. D. Bond and A. B. Holmes, Chem. Commun., 2002, 2424 RSC.
- J. K. Lee, S. Lee and S. J. Yun, Bull. Korean Chem. Soc., 2013, 34, 2148 CrossRef CAS.
- T.-H. Kwon, V. Armel, A. Nattestad, D. R. MacFarlane, U. Bach, S. J. Lind, K. C. Gordon, W. Tang, D. J. Jones and A. B. Holmes, J. Org. Chem., 2011, 76, 4088 CrossRef CAS PubMed.
- (a) J. Kwon, T.-H. Kwon, H. S. Cho, M. K. Kim, I.-S. Shin, D.-Y. Shin, S. J. Park and J.-I. Hong, New J. Chem., 2008, 32, 1368 RSC; (b) M. K. Kim, J. Kwon, T.-H. Kwon and J.-I. Hong, New J. Chem., 2010, 34, 1317 RSC.
- M. D. Perez, C. Borek, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2009, 131, 9281 CrossRef CAS PubMed.
- (a) M. Zhang, H. Wang, H. Tian, Y. Geng and C. W. Tang, Adv. Mater., 2011, 23, 4960 CrossRef CAS PubMed; (b) Y.-q. Zheng, W. J. Potscavage, Jr, T. Komino, H. Hirade, J. Adachi and C. Adachi, Appl. Phys. Lett., 2013, 102, 143304 CrossRef PubMed.
- (a) I. Riedel, J. Parisi, V. Dyakonov, L. Lutsen, D. Vanderzande and J. C. Hummelen, Adv. Funct. Mater., 2004, 14, 38 CrossRef CAS; (b) L. J. A. Koster, V. D. Mihailetchi, H. Xie and P. W. M. Blom, Appl. Phys. Lett., 2005, 87, 203502 CrossRef PubMed; (c) V. D. Mihailetchi, J. Wildeman and P. W. M. Blom, Phys. Rev. Lett., 2005, 94, 126602 CrossRef CAS.
- J.-Y. Pan, L.-J. Zuo, X.-L. Hu, W.-F. Fu, M.-R. Chen, L. Fu, X. Gu, H.-Q. Shi, M.-M. Shi, H.-Y. Li and H.-Z. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 972 CAS.
Footnotes |
| † Electronic supplementary information available: Detailed experimental procedures, photophysical data, thermal data, electrochemical data, XRD data, device data, and AFM data, additional supporting data. See DOI: 10.1039/c4ra02895c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |





