Formation of a gold–carbon dot nanocomposite with superior catalytic ability for the reduction of aromatic nitro groups in water†
Abstract
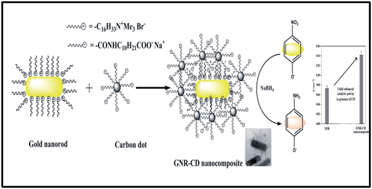
We report the synthesis of a gold–carbon dot nanocomposite and its utility as a recyclable catalyst for the reduction of aromatic nitro groups. The presence of carbon dots on gold nanosurfaces enhanced the reduction rate by two-fold.

 Please wait while we load your content...
Please wait while we load your content...