Graphene oxide based BCNO hybrid nanostructures: tunable band gaps for full colour white emission†
Abstract
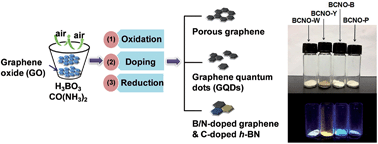
The emission of BCNO phosphors has been easily tuned from the violet to the near red regions by varying the carbon content. Here we report the optimal conversion of graphene oxide (GO) into BCNO hybrid nanostructures by one-step air oxidation with boric acid and urea. White lighting phosphor was obtained in which the doped porous graphene acts as an interconnecting framework generating and transferring electrons under excitation light. Various carbon-related levels in the BN band structures play an essential role in emitting full colour white light. The quantum confinement in the various kinds of GQDs and GO are also beneficial to widen the emission spectrum.

 Please wait while we load your content...
Please wait while we load your content...