An azafullerene acceptor for organic solar cells†
Zuo Xiao‡
a,
Dan He‡a,
Chuantian Zuoa,
Liangbing Gan*bc and
Liming Ding*a
aNational Center for Nanoscience and Technology, Beijing 100190, China. E-mail: opv.china@yahoo.com
bBeijing National Laboratory for Molecular Sciences (BNLMS), Beijing 100080, China
cKey Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: gan@pku.edu.cn
First published on 23rd May 2014
Abstract
A novel azafullerene derivative, OQThC59N, was prepared and used as the acceptor material in solution-processed bulk heterojunction organic solar cells. Possessing a relatively high LUMO level, OQThC59N gives a high open-circuit voltage of 0.78 V and a power conversion efficiency of 4.09% in fullerene:P3HT solar cells.
Azafullerenes are novel carbon clusters with one or more fullerene cage carbons replaced by nitrogen atoms.1 Wudl et al. reported the first bulk preparation of structurally defined azafullerenes, (C59N)2 and RC59N, via a cage-opened fullerene ketolactam.2 Hirsch et al. also succeeded in the synthesis of (C59N)2 and RC59N using a bisazafulleroid as the starting material.3 Gan et al. prepared azafullerene derivatives with peroxide addends, R4XC59N (R = tBuOO, X = H, OH, or Br).4 Fullerenes and their derivatives have been used as acceptor materials in organic solar cells (OSCs) due to their high electron affinity, unique sphericity, and excellent electron-transporting properties.5 The power conversion efficiency of bulk heterojunction (BHJ) OSCs containing a conjugated polymer donor and a fullerene acceptor has surpassed 9%.6 Since nitrogen contains one more electron than carbon does, replacing C by N in fullerene can be viewed as a further n-doping to fullerene.7 Solar cells using azafullerenes have not been reported yet. In this work, an azafullerene derivative (OQThC59N) was synthesized and used as the acceptor material in OSCs. OQThC59N possesses a lower optical bandgap and a higher LUMO level than [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM). OQThC59N shows better performance than PC61BM in fullerene:P3HT solar cells.
We first prepared mono-arylated azafullerene ThC59N following Hirsch's one-pot method.8 Heating fullerene ketolactam 1 in the presence of air and excessive p-toluenesulfonic acid (p-TsOH) generated azafullerenium carbocation C59N+, which was then trapped by electron-rich thiophene to give green ThC59N in 22% yield (Scheme 1). 1H NMR spectrum showed three peaks at 8.32, 7.80, and 7.50 ppm, respectively, corresponding to three aromatic H of thiophene. 13C NMR spectrum showed 34 peaks for sp2 carbons ranging from 123.66 to 153.49 ppm and one peak at 99.36 ppm for one sp3 carbon on fullerene, which is consistent with the Cs symmetry of ThC59N.9 To obtain solution-processable acceptors, we further modified ThC59N with o-quinodimethane diene through a Diels–Alder reaction.10 OQThC59N was obtained in 36% yield. OQThC59N consists of regioisomers as indicated by NMR. On 1H NMR spectrum of OQThC59N, the integral ratio between aromatic H (7.34–8.46 ppm) and aliphatic H (3.73–4.98 ppm) is 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, indicating that only 1 equiv. diene was added to ThC59N. High resolution ESI mass spectrum for OQThC59N showed the expected molecular ion peak (M + H+) at 910.0704 m/z. Compared with ThC59N, OQThC59N shows a good solubility in organic solvents, e.g. 37 mg mL−1 in o-dichlorobenzene (ODCB).
4, indicating that only 1 equiv. diene was added to ThC59N. High resolution ESI mass spectrum for OQThC59N showed the expected molecular ion peak (M + H+) at 910.0704 m/z. Compared with ThC59N, OQThC59N shows a good solubility in organic solvents, e.g. 37 mg mL−1 in o-dichlorobenzene (ODCB).
Optical and electrochemical properties of azafullerenes ThC59N and OQThC59N were studied and compared with that of PC61BM. Azafullerenes showed broader absorption extending to ∼850 nm (Fig. 1). Optical bandgaps estimated from absorption onsets are 1.45 and 1.47 eV for ThC59N and OQThC59N, respectively, which are much smaller than that of PC61BM (1.71 eV). ThC59N showed four characteristic absorption peaks at 319, 443, 715, and 799 nm, respectively, which are typical for mono-substituted azafullerenes.9 OQThC59N showed lower absorbance in UV region but higher absorbance in both visible and NIR regions than PC61BM, suggesting that OQThC59N could produce higher photocurrent in OSCs.11 The LUMO levels of ThC59N, OQThC59N, and PC61BM were estimated from their first reduction potentials (E1) by using the empirical equation, LUMO = −(E1 + 4.8) eV (Fig. 2). Compared with PC61BM's LUMO (−3.67 eV), the LUMO level for ThC59N (−3.72 eV) slightly drops, while that for OQThC59N (−3.56 eV) hoists. 0.16 eV hoist in LUMO level from ThC59N to OQThC59N results from the shrinkage of fullerene π system.10c High LUMO level favors to enhance Voc.12
Solar cells with a structure of ITO/PEDOT:PSS/fullerene:P3HT/Ca/Al were studied. Donor/acceptor (D/A) ratio, film thickness, and annealing temperature were optimized for OQThC59N cells (Table S1–S3†). OQThC59N cells with a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
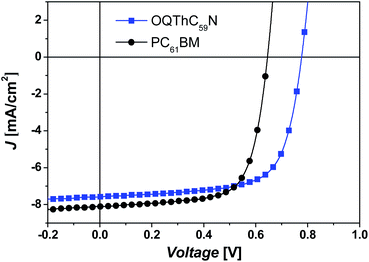
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 (w/w), an active layer thickness of ∼100 nm, being annealed at 110 °C for 10 min afforded the best results, giving a Voc of 0.78 V, a Jsc of 7.57 mA cm−2, a FF of 69.2%, and a PCE of 4.09%. Under the same conditions, PC61BM cells afforded a lower PCE of 3.67%, with a Voc of 0.65 V, a Jsc of 8.10 mA cm−2, and a FF of 69.8% (Fig. 3 and Table 1). Obviously, the better performance of OQThC59N cells benefits from Voc, which is 0.13 V higher than that of PC61BM cells. The increase in Voc is consistent with higher LUMO of OQThC59N. OQThC59N cells showed lower external quantum efficiency (EQE) than PC61BM cells in 400–650 nm region, corresponding with the lower Jsc of OQThC59N cells (Fig. S5†). The expected stronger film absorption and EQE response in the long wavelength region was not observed for OQThC59N cells (Fig. S6†), which might be due to small absorption coefficient of OQThC59N in this region.
0.6 (w/w), an active layer thickness of ∼100 nm, being annealed at 110 °C for 10 min afforded the best results, giving a Voc of 0.78 V, a Jsc of 7.57 mA cm−2, a FF of 69.2%, and a PCE of 4.09%. Under the same conditions, PC61BM cells afforded a lower PCE of 3.67%, with a Voc of 0.65 V, a Jsc of 8.10 mA cm−2, and a FF of 69.8% (Fig. 3 and Table 1). Obviously, the better performance of OQThC59N cells benefits from Voc, which is 0.13 V higher than that of PC61BM cells. The increase in Voc is consistent with higher LUMO of OQThC59N. OQThC59N cells showed lower external quantum efficiency (EQE) than PC61BM cells in 400–650 nm region, corresponding with the lower Jsc of OQThC59N cells (Fig. S5†). The expected stronger film absorption and EQE response in the long wavelength region was not observed for OQThC59N cells (Fig. S6†), which might be due to small absorption coefficient of OQThC59N in this region.
Electron mobility of fullerene was measured by space charge limited current (SCLC) method. The mobility was determined by fitting the dark current to the model of a single carrier SCLC.13 Fig. 4(a) shows J–V curves for the electron-only devices. The mobility was calculated from the slope of J1/2–V lines. OQThC59N possesses a lower electron mobility of 8.9 × 10−5 cm2 V−1 s−1 than that of PC61BM (3.0 × 10−4 cm2 V−1 s−1). Atomic force microscope (AFM) indicates that OQThC59N:P3HT blend film is coarser than PC61BM:P3HT blend film (Fig. 5). The root-mean-square (RMS) roughnesses for OQThC59N:P3HT and PC61BM:P3HT blend films are 2.6 and 1.4 nm, respectively, suggesting that OQThC59N might possess lower miscibility with P3HT than PC61BM. Lower mobility of OQThC59N might account for lower Jsc of OQThC59N cells.
 | ||
| Fig. 4 J–V curves (a) and the corresponding J1/2–V curves (b) for electron-only devices (in dark). The thicknesses for OQThC59N:P3HT and PC61BM:P3HT blend films are 110 nm and 101 nm, respectively. | ||
In summary, for the first time we have introduced an azafullerene, OQThC59N, as an acceptor used in OSCs. The 4.09% PCE and 0.78 V Voc given by OQThC59N:P3HT solar cells demonstrate the potential of azafullerenes. There is plenty of room for developing efficient azafullerene acceptors by improving the mobility and the miscibility with donor materials.
Acknowledgements
This work was supported by the “100 Talents Program” of Chinese Academy of Sciences and National Natural Science Foundation of China (21374025, 21372053 and 21102028). Z. X. appreciates the support from Beijing National Laboratory for Molecular Sciences. We thank Gang Ye and Shan Chen for technical assistance.Notes and references
- O. Vostrowsky and A. Hirsch, Chem. Rev., 2006, 106, 5191 CrossRef CAS PubMed.
- (a) J. C. Hummelen, B. Knight, J. Pavlovich, R. Gonzalez and F. Wudl, Science, 1995, 269, 1554 CAS; (b) M. Keshavarz-K, R. Gonzalez, R. G. Hicks, G. Srdanov, V. I. Srdanov, T. G. Collins, J. C. Hummelen, C. Bellavia-Lund, J. Pavlovich, F. Wudl and K. Holczer, Nature, 1996, 383, 147 CrossRef CAS.
- B. Nuber and A. Hirsch, Chem. Commun., 1996, 1421 RSC.
- G. Zhang, S. Huang, Z. Xiao, Q. Chen, L. Gan and Z. Wang, J. Am. Chem. Soc., 2008, 130, 12614 CrossRef CAS PubMed.
- (a) Y. Li, Chem.–Asian J., 2013, 8, 2316 CrossRef CAS PubMed; (b) C.-Z. Li, H. L. Yip and A. K. Y. Jen, J. Mater. Chem., 2012, 22, 4161 RSC; (c) F. G. Brunetti, R. Kumar and F. Wudl, J. Mater. Chem., 2010, 20, 2934 RSC; (d) J. L. Delgado, P. A. Bouit, S. Filippone, M. A. Herranz and N. Martin, Chem. Commun., 2010, 46, 4853 RSC; (e) B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef CAS PubMed; (f) C. L. Chochos, N. Tagmatarchis and V. G. Gregoriou, RSC Adv., 2013, 3, 7160 RSC.
- (a) Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 Search PubMed; (b) S.-H. Liao, H.-J. Jhuo, Y.-S. Cheng and S.-A. Chen, Adv. Mater., 2013, 25, 4766 CrossRef CAS PubMed.
- (a) P. Ayala, R. Arenal, M. Rümmeli, A. Rubio and T. Pichler, Carbon, 2010, 48, 575 CrossRef CAS PubMed; (b) Y. Li, T. Kaneko, S. Miyanaga and R. Hatakeyama, ACS Nano, 2010, 4, 3522 CrossRef CAS PubMed; (c) Y. Li, T. Kaneko, J. Kong and R. Hatakeyama, J. Am. Chem. Soc., 2009, 131, 3412 CrossRef CAS PubMed.
- B. Nuber and A. Hirsch, Chem. Commun., 1998, 405 RSC.
- F. Hauke, O. Vostrowsky, A. Hirsch, A. Quaranta, W. Leibl, S. Leach, R. Edge, S. Navaratnam and R. V. Bensasson, Chem.–Eur. J., 2006, 12, 4813 CrossRef CAS PubMed.
- (a) X. Meng, W. Zhang, Z. Tan, C. Du, C. Li, Z. Bo, Y. Li, X. Yang, M. Zhen, F. Jiang, J. Zheng, T. Wang, L. Jiang, C. Shu and C. Wang, Chem. Commun., 2012, 48, 425 RSC; (b) G. Ye, S. Chen, Z. Xiao, Q. Zuo, Q. Wei and L. Ding, J. Mater. Chem., 2012, 22, 22374 RSC; (c) D. He, X. Du, Z. Xiao and L. Ding, Org. Lett., 2014, 16, 612 CrossRef CAS PubMed; (d) D. He, X. Du, W. Zhang, Z. Xiao and L. Ding, J. Mater. Chem. A, 2013, 1, 4589 RSC; (e) S. Chen, G. Ye, Z. Xiao and L. Ding, J. Mater. Chem. A, 2013, 1, 5562 RSC; (f) C. Zhang, S. Chen, Z. Xiao, Q. Zuo and L. Ding, Org. Lett., 2012, 14, 1508 CrossRef CAS PubMed; (g) D. He, C. Zuo, S. Chen, Z. Xiao and L. Ding, Phys. Chem. Chem. Phys., 2014, 16, 7205 RSC.
- Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038 CrossRef CAS PubMed.
- B. P. Rand, D. P. Burk and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 115327 CrossRef.
- Z. He, C. Zhong, X. Huang, W. Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details including synthesis, measurements, and instruments. See DOI: 10.1039/c4ra02757d |
| ‡ Z. Xiao and D. He contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |