Flexible aerogels with interpenetrating network structure of bacterial cellulose–silica composite from sodium silicate precursor via freeze drying process†
Abstract
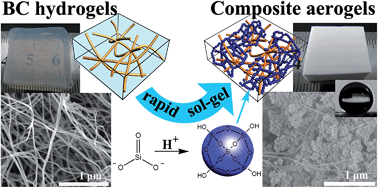
Bacterial cellulose (BC)–silica composite aerogels (CAs) with interpenetrating network (IPN) microstructure are prepared through a permeation sol–gel process followed by freeze drying. The IPN structure is constructed by diffusing the precursor into a three-dimensional (3D) BC matrix followed by permeating the catalyst into the BC network gradually to promote the in situ condensation of precursor to form a SiO2 gel skeleton from outside to inside. The precursor used here is Na2SiO3 instead of traditional tetraethoxysilane. This IPN structure could offer excellent mechanical properties to aerogels, and is essential to prepare flexible aerogels by freeze drying. The compression modulus of CAs could be adjusted in the range of 0.38 MPa to 16.17 MPa. The BC–silica CAs exhibit low density (as low as 0.011 g cm−3), high specific surface area (as high as 534.5 m2 g−1) and low thermal conductivity (less than 0.0369 W m−1 K−1). Furthermore, the contact angle of the hydrophobization modified CAs is as high as 145°. The outstanding hydrophobicity and the large specific surface area endow the hydrophobic CAs with excellent oil absorption capability on the water surface. Moreover, the hydrophobic CAs that had absorbed oil could be washed and recycled.

 Please wait while we load your content...
Please wait while we load your content...