A highly selective visual detection of tabun mimic diethyl cyanophosphate (DCNP): effective discrimination of DCNP from other nerve agent mimics†
D. Raghavender Goud,
Deepak Pardasani,
Vijay Tak and
Devendra Kumar Dubey*
Vertox Laboratory, Defence Research and Development Establishment, Jhansi Road, Gwalior, M.P. 474002, India. E-mail: dkdubey@rediffmail.com; Fax: +91-0751-2341148
First published on 23rd May 2014
Abstract
A new tabun specific visual detection protocol is reported. The chemodosimeter fluorescein-hydroxamate aldehyde undergoes tandem nucleophilic substitution followed by cyanohydrin reaction with DCNP and shows a specific spectroscopic behaviour. The probe molecule clearly distinguishes tabun mimic DCNP over other nerve agent simulants as well as strong electrophiles.
Nerve agents are the most toxic of the known chemical warfare agents. These agents are hazardous and can cause death within minutes after exposure. The ease of production, extreme toxicity and the current rise in international concern over the use of chemical warfare agents in terrorism, accentuates the need to develop simple and reliable methods to detect these lethal chemicals.1 Nerve agents inhibit the enzyme acetylcholinesterase (AChE), which degrades the neurotransmitter acetylcholine. This leads to accumulation of acetylcholine at nerve synapses resulting neuromuscular paralysis and eventually death.2 Various methods for the detection of nerve agents have been developed including enzymatic assays,3 optical-fiber arrays,4 surface acoustic wave (SAW) devices,5 electrochemistry,6 biosensors7 and ion mobility spectroscopy.8 However, they show at least one of the following limitations; operational complexity, non-portability, high cost, difficulties in real-time monitoring and false positive readings.
In recent years development of chromogenic and fluorescence detection methods have gained considerable interest as an alternative to above mentioned complex methods. The main advantages of chromogenic detection method are portability, easy to operate, cheap and capable of real time detection. The recently developed methods are PET based probes,9 cyclisation reactions10 in push–pull chromophores, displacement like assays,11 molecular impregnated polymers,12 oximate containing sensors,13 nucleophilic substitution reactions14 and complex formation based probes.15 The main drawback of these methods is lack of specificity towards the target analyte. Most of these methods mainly target the electrophilicity of nerve agents, therefore the other reactive electrophiles also give the similar response as nerve agents. Although the disaster response protocol is similar for all nerve agents, but the choice of oxime for AChE enzyme reactivation is not same. Experimental evidences shows that PAM is preferred for sarin and VX, HI-6 for soman and obidoxime for tabun.16 These reasons emphasise the need for the development of specific detection methods for each nerve agent.
Herein, we report a tabun specific chemodosimeter molecule that is able to differentiate DCNP (tabun mimic) from other nerve agent mimics as well as strong electrophiles. The main structural difference among the tabun and other nerve agents or their mimics is the electrophilic phosphorous centre is bonded to cyanide anion in tabun. Whereas in case of other nerve agents the anions other than cyanide like fluoride, thiolate and chloride were bonded to electrophilic phosphorous centre of the nerve agents (Fig. 1). Therefore based on these observations we expected that specific colorimetric detection of the tabun is possible by targeting both the electrophilic character and cyanide anion present in tabun. In this study we have chosen DCNP as a tabun mimic as it behaves similar to tabun due to the presence of electrophilic phosphorous centre is attached to cyanide anion but less toxic than tabun.
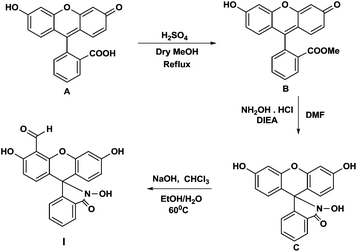
The tabun specific chemodosimeter molecule fluorescein-hydroxamate aldehyde (I) was designed in such a way that it consist of two reactive sites (Scheme 1). One is oximate group that acts as a nucleophile and is reactive towards the electrophilic phosphorous centre of the nerve agents. Another reactive site is aldehyde group that acts as an electrophile and selectively reacts with the cyanide anion released from DCNP via cyanohydrin formation. This cascade reaction may result in the specific optical behaviour towards DCNP.
The probe molecule was synthesized by a three step procedure. In the first step fluorescein methyl ester was synthesized by Fischer esterification of fluorescein. In the next step fluorescein hydroxamate was synthesized by nucleophilic substitution with hydroxylamine hydrochloride. Finally the target molecule was synthesized by introducing the aldehyde group by Riemer–Tiemann reaction (Scheme 1).
To test selectivity of the probe molecule, the reactions of probe molecule with DCNP (tabun mimic) and DCP (nerve agent mimic) were screened in various solvents. Among these DMF and DMSO were optimized for DCP and DCNP respectively. The optimized test solution consists of the chemodosimeter (1 mg ml−1) and 3% (v/v) triethylamine as a base. With DCP the probe molecule gives intense red colour in DMF, where as in DMSO it gives weak orange-red colour. This may be due to the negative solvatochromic effect.17 Therefore better sensitivity can be achieved for DCP in DMF when compared to DMSO. Due to this reason DMF was preferred for DCP analysis. In case of DCNP 5 mg of KI was used as a catalyst. As DCNP is less reactive than DCP, iodide by virtue of its polarisable nucleophilicity and nucleofugicity facilitates the phosphorylation of (I) by efficiently knocking out the cyanide present in DCNP. KI doesn't interfere with the detection of DCP. Initially the test solutions were yellow in colour and exhibit a unique band at 410 nm (Fig. 2). When a nerve agent simulant DCP (10 equivalents) was added to the test solution a remarkable colour modulation from yellow to red was observed. When DCP reacts with the chemodosimeter molecule, the hydroxamate group of the probe molecule reacts at electrophilic phosphorous centre of the nerve agent mimic via nucleophilic substitution to give an intermediate (IIA). In the next step the intermediate (IIA) undergoes Lossen rearrangement to afford conjugated product (IIIA), which is responsible for the bathochromic shift to 530 nm (Fig. 2). Similarly by the addition of tabun mimic DCNP (10 equivalents) to the test solution, colour change to blue-green was observed. When DCNP reacts with the probe molecule, the hydroxamate group of the probe molecule reacts at electrophilic phosphorous centre of DCNP and subsequently the released cyanide anion undergoes electrophilic addition reaction (cyanohydrin formation) with aldehyde group to give intermediate (IIB). In the next step the intermediate undergoes Lossen rearrangement to result in the formation of another highly conjugated product (IIIB) and there was a further bathochromic shift to 608 nm (Fig. 2) was observed which is responsible for the blue-green colour. In product (IIIA) the delocalisation of conjugated electrons was retarded due to the presence of highly electron withdrawing aldehyde group (Scheme 2).
 | ||
| Fig. 2 UV/Vis spectra of chemodosimeter (I) (C = 1 mM) solution in the presence of DCP and DCNP (10 equivalents). | ||
 | ||
| Scheme 2 Chromogenic response mechanism for chemodosimeter (I) in the presence of nerve agent simulants DCP and DCNP. | ||
Where as in case of product (IIIB) due to the formation of cyanohydrin the electron withdrawing effect of aldehyde group is lost, this increases the degree of delocalisation in the product (IIIB) which may be responsible for further bathochromic shift. The reaction of fluorescein-hydroxamate aldehyde with DCNP and DCP was monitored by subjecting the reaction solution to ESI-MS analysis. The peaks corresponding to the molecular weight of the intermediates and the products were identified, this confirms the formation of these products in assay systems (See ESI, Fig. S6 and S7†).
In the next step, to test the interferences the chemodosimeter molecule was reacted with various strong electrophiles like thionyl chloride, phosphorous oxychloride, 4-toluenesulfonyl chloride and acetyl chloride. In all the cases instantaneous colour change to red was observed similar to DCP as shown in the Fig. 3. In addition to this, interferences of anions like cyanide, iodide, bromide and fluoride were also checked. No change in spectroscopic behaviour was noted in the presence of any of these anions (Fig. 3). Only with DCNP (10 equivalent) specific colour change to blue-green was observed (Fig. 3), which indicates that the probe molecule clearly distinguishes the tabun mimic DCNP over other electrophiles and nerve agent mimics. The chemodosimeter (I) was also checked for fluorescence response against DCP and DCNP. The probe molecule was found to be fluorescent for DCP but non-fluorescent for DCNP.
To test the sensitivity of the chemodosimeter towards tabun mimic DCNP and nerve agent mimic DCP, the analytes with various concentrations were added to their respective reagent solutions. In case of DCP the colour change to red was instantaneous at room temperature, the reactions were incubated for 15 min and absorbance was recorded. DCP with detection limit 0.15 mM can be clearly identified. Whereas in case of DCNP initially the solution became colourless then the blue-green colour intensified slowly. The reactions were incubated for 1 h at room temperature before measuring the absorbance, a minimum detection limit for DCNP 3 mM can be achieved by the heating the solution at 60 °C for 15 min (see ESI†). However different products were formed during the course of reaction in both the cases, the chromophore responsible for absorbance was same in all the intermediates and products. So the colour developed in each case remains stable, no change in λmax was observed even after 12 h at room temperature. However storing for more than 3 to 7 days intensity of DCNP diminished with the formation of intricate reaction mixture. The chromogenic response was found to be reproducible for both the analytes.
In summary, a visual and highly selective detection method for tabun mimic DCNP was developed by targeting the electrophilicity as well as the presence of cyanide anion in DCNP. The probe molecule fluorescein-hydroxamate aldehyde undergoes tandem phosphorylation followed by cyanohydrin reaction with DCNP to afford a specific product, which shows the specific spectroscopic behaviour. This specific product formation takes place only in the presence of DCNP, therefore no false positives were noted for all possible closely related interferences. We have successfully demonstrated the possibility of detecting tabun mimic DCNP using a tandem reaction based probe molecule. The similar approach can be used to develop the probe molecules for the selective detection of other nerve agents.
Notes and references
- (a) H. H. Hill Jr and S. J. Martin, Pure Appl. Chem., 2002, 74, 2281 Search PubMed; (b) M. Wheelis, Pure Appl. Chem., 2002, 74, 2247 CrossRef CAS.
- (a) T. C. Marrs, Pharmacol. Ther., 1993, 58, 51 CrossRef CAS; (b) F. R. Sidell and J. Borak, Ann. Emerg. Med., 1992, 21, 865 CrossRef CAS.
- A. J. Russel, J. A. Berberich, G. F. Drevon and R. R. Koepsel, Annu. Rev. Biomed. Eng., 2003, 5, 1 CrossRef PubMed.
- (a) M. T. McBride, S. Gammon, M. Pitesky, T. W. O. Brien, T. Smith, J. Aldrich, R. G. Langlois, B. Colston and K. S. Venkateswaran, Anal. Chem., 2003, 75, 1924 CrossRef CAS; (b) L. Song, S. Ahn and D. R. Walt, Anal. Chem., 2006, 78, 1023 CrossRef CAS PubMed.
- (a) M. S. Nieuwenhuizen and J. L. N. Harteveld, Sens. Actuators, B, 1997, 40, 167 CrossRef CAS; (b) J. Negeh-Ngwainbi, P. H. Foley, S. S. Kuan and G. G. Guilbault, J. Am. Chem. Soc., 1986, 108, 5444 CrossRef; (c) Y. Yang, H. F. Hi and T. Thundat, J. Am. Chem. Soc., 2003, 125, 1124 CrossRef CAS PubMed; (d) C. Hartmann-Thompson, J. Hu, S. N. Kaganove, S. E. Keinath, D. L. Keeley and P. R. Dvornic, Chem. Mater., 2004, 16, 5357 CrossRef CAS.
- (a) Y. Lin, F. Lu and J. Wang, Electroanalysis, 2004, 16, 145 CrossRef CAS; (b) Y. Zhou, B. Yu, E. Shiu and K. Levon, Anal. Chem., 2004, 76, 2689 CrossRef CAS PubMed; (c) D. Yu, J. Volponi, S. Chhabra, C. J. Brinker, A. Mulchandani and A. K. Singh, Biosens. Bioelectron., 2005, 20, 1433 CrossRef CAS PubMed; (d) K. Anitha, S. V. Mohan and S. J. Reddy, Biosens. Bioelectron., 2004, 19, 848 CrossRef PubMed; (e) A. L. Simonian, J. K. Grimsley, A. W. Flounders, J. S. Schoeniger, C. T. Cheng, J. J. DeFrank and J. R. Wild, Anal. Chim. Acta, 2001, 442, 15 CrossRef CAS; (f) M. H. Hammond, K. J. Johnson, S. L. Rose Pehrsson, J. Ziegler, H. Walker, K. Coudy, D. Gary and D. Tillett, Sens. Actuators, B, 2006, 116, 135 CrossRef CAS PubMed; (g) J. Wang, Anal. Chim. Acta, 2004, 507, 3 CrossRef CAS PubMed.
- (a) J. J. Gooding, Anal. Chim. Acta, 2006, 559, 137 CrossRef CAS PubMed; (b) F. Arduini, A. Amine, D. Moscone and G. Palleschi, Microchim. Acta, 2010, 170, 193 CrossRef CAS.
- (a) R. A. Miller, E. G. Nazarov, G. A. Eiceman and A. T. King, Sens. Actuators, A, 2001, 91, 301 CrossRef CAS; (b) K. Tuovinen, H. Paakkanen and O. Hanninen, Anal. Chim. Acta, 2001, 440, 151 CrossRef CAS.
- (a) K. A. Van Houten, D. C. Heath and R. S. Pilato, J. Am. Chem. Soc., 1998, 120, 12359 CrossRef CAS; (b) S. W. Zhang and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 3420 CrossRef CAS PubMed; (c) T. J. Dale and J. Rebek Jr, J. Am. Chem. Soc., 2006, 128, 4500 CrossRef CAS PubMed; (d) F. Ilhan, D. S. Tyson and M. A. Meador, Chem. Mater., 2004, 16, 2978 CrossRef CAS; (e) S. Bencic-Nagale, T. Sternfeld and D. R. Walt, J. Am. Chem. Soc., 2006, 128, 5041 CrossRef CAS PubMed.
- (a) A. M. Costero, S. Gil, M. Parra, P. M. E. Mancini, R. Martínez-Máñez, F. Sancenón and S. Royo, Chem. Commun., 2008, 6002 RSC; (b) A. M. Costero, M. Parra, S. Gil, R. Gotor, P. M. E. Mancini, R. Martínez-Máñez, F. Sancenón and S. Royo, Chem.–Asian J., 2010, 5, 1573 CrossRef CAS PubMed.
- D. Knapton, M. Burnworth, S. J. Rowan and C. Weder, Angew. Chem., Int. Ed., 2006, 45, 5825 CrossRef CAS PubMed.
- (a) A. L. Jenkins, O. M. Uy and G. M. Murray, Anal. Commun., 1997, 34, 221 RSC; (b) A. L. Jenkins, O. M. Uy and G. M. Murray, Anal. Chem., 1999, 71, 373 CrossRef CAS; (c) A. L. Jenkins and S. Y. Bae, Anal. Chim. Acta, 2005, 542, 32 CrossRef CAS PubMed; (d) G. E. Southard, K. A. Van Houten, E. W. Ott Jr and G. M. Murray, Anal. Chim. Acta, 2007, 581, 202 CrossRef CAS PubMed.
- (a) K. J. Wallace, R. I. Fagbemi, F. J. Folmer-Andersen, J. Morey, V. M. Lynch and E. V. Anslyn, Chem. Commun., 2006, 3886 RSC; (b) F. Terrier, P. Rodríguez-Dafonte, E. Le Guével and G. Moutiers, Org. Biomol. Chem., 2006, 4, 4352 RSC.
- (a) S. Han, Z. Xue, Z. Wang and T. B. Wen, Chem. Commun., 2010, 46, 8413 RSC; (b) W. Xuan, Y. Cao, J. Zhou and W. Wang, Chem. Commun., 2013, 49, 10474 RSC; (c) H. J. Kim, S. Jang, W. X. Ren, R. A. Bartsch, H. Sohn and J. S. Kim, Sens. Actuators, B, 2011, 153, 266 CrossRef CAS PubMed; (d) A. J. Weerasinghe, C. Schmiesing and E. Sinn, Tetrahedron, 2011, 67, 2833 CrossRef CAS PubMed; (e) Z. Wu, X. Wu, Y. Yang, T.-B. Wen and S. Han, Bioorg. Med. Chem. Lett., 2012, 22, 6358 CrossRef CAS PubMed; (f) K. Chulvi, P. Gaviña, A. M. Costero, S. Gil, M. Parra, R. Gotor, S. Royo, R. Martínez-Máñez, F. Sancenón and J. Vivancos, Chem. Commun., 2012, 48, 10105 RSC; (g) K. L. Diehl and E. V. Anslyn, Chem. Soc. Rev., 2013, 42, 8596 RSC.
- (a) M. R. Sambrook, J. R. Hiscock, A. Cook, A. C. Green, I. Holden, J. C. Vincent and P. A. Gale, Chem. Commun., 2012, 48, 5605 RSC; (b) A. Wild, A. Winter, M. D. Hager and U. S. Schubert, Chem. Commun., 2012, 48, 964 RSC.
- Handbook of Toxicology of Chemical Warfare Agents, ed. R. C. Gupta, Elsevier, London, 2009, p. 27 Search PubMed.
- H. M. Reza, C. M. Javad and Y. Maryam, Iran. J. Chem. Chem. Eng., 2008, 27, 9 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional characterization data. See DOI: 10.1039/c4ra02742f |
| This journal is © The Royal Society of Chemistry 2014 |