One-pot synthesis of 5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives†
Hong-Ru Donga,
Zhong-Lian Gaoa,
Rong-Shan Lia,
Yi-Ming Hub,
Heng-Shan Dong*a and
Zhi-Xiang Xiea
aCollege of Chemistry and Chemical Engineering, State Key Laboratory of Applied Organic Chemistry, Institute of Organic Chemistry, Lanzhou University, Lanzhou, Gansu 730000, P. R. China. E-mail: donghengshan@lzu.edu.cn; xiezx@lzu.edu.cn
bThe School of Nuclear Science and Technology, Lanzhou University, Lanzhou, Gansu 730000, P. R. China
First published on 7th October 2014
Abstract
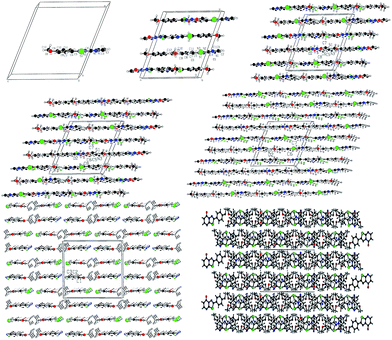
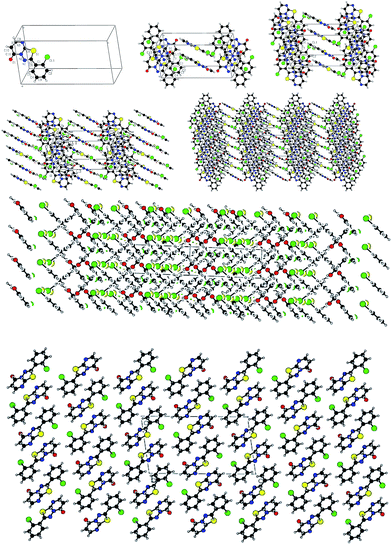
A novel and efficient one-pot method has been developed for the synthesis of 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivative by the combination of [3 + 3] cycloaddition, reduction, deamination reactions. The fused heterocyclic compounds 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-ones was synthesized by the diversity-oriented catalysis. As an extension of the synthetic methodology, some 4H-pyrido[1,2-a]pyrimidin-4-one 4a–c was synthesized. The π–π accumulation structure of supramolecular self-assembly was discussed in the crystal.
Thiadiazolopyrimidines, analogous structure compounds display an important role in pharmacological chemistry. The nucleus and a number of its substituted products exhibit interesting biological and pharmacological properties.1–10 Because of their importance; the synthesis of these compounds has attracted considerable attention. In recent years, many papers concerning the synthesis of 2,7-disubstituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives have been reported.11–15 However, little attention was paid to the synthesis of 2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-ones, which are unsubstituted on the 7-position and till now, there are few reports of their synthesis in literature. The study of the methodology of their synthesis and structure, and the structure of their derivatives and the analogous compounds in biological and pharmacological fields, is important.
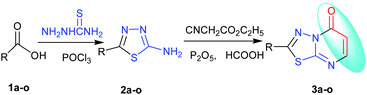
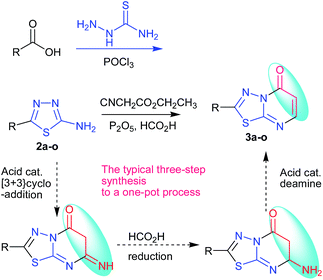
The two-steps synthesis method of 2-amino-7-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives have been reported, which were prepared from 2-amino-thiadiazole derivatives and β-keto esters, but the yield was low.13,14 Cascade, tandem, one-pot, multicomponent domino reactions, atom economy, catalyst-free, ring opening, supramolecular self-assembly and diversity-oriented synthesis (DOS) are increasingly applied to the preparation of natural and designed molecules.16 A novel and efficient one-pot method has been developed for the synthesis of 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivative. Some novel fused-ring systems 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives 3a–o were synthesized by the one-pot reaction of the corresponding 2-amino-5-substitued-1,3,4-thiadiazoles 2a–o and ethyl cyanoacetate under the presence of catalytic agent (Scheme 1).
 | ||
| Scheme 1 The synthesis of 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives for combination of [3 + 3] cycloaddition, reduction, deamination. | ||
When a solution of 2-amino-5-substituted-1,3,4-thiadiazole, ethyl cyanoacetate and CH3ONa in dry methanol was refluxed, the 2-cyano-N-(5-substituted-1,3,4-thiadiazol-2-yl)acetamide 2 was obtained.17 Previously, when the solution of 2-amino-5-substituted-1,3,4-thiadiazole, ethyl cyanoacetate and CH3ONa in dry methanol was refluxed, the reaction gave another compound, 5,6-dihydro-5-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-7-one18 3. The same type of reaction gave different results. The ring conversion reaction of 5,6-dihydro-5-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-7-one in formic acid at 100 °C for 10 h was carried out to produce 6,7-dihydro-7-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one 4.19 The reaction of 2-amino-5-substituted-1,3,4-thiadiazole, ethyl cyanoacetate and methanesulfonic acid–phosphorus pentoxide gave 6,7-dihydro-7-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one 4.16 With the same starting materials, reagents, solvents and catalytic agent, the reaction gave different products to the intermediate 2-cyano-N-(5-substituted-1,3,4-thiadiazol-2-yl)acetamide 2 or 5,6-dihydro-5-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-7-one 3. With the same starting materials and reagents, the reaction can also give the same products using different catalytic agents. 6,7-Dihydro-7-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one 4 could be synthesized by 2–3 steps or one step from 2-amino-5-substituted-1,3,4-thiadiazole. The diversity of the synthesis is shown in Scheme 2.
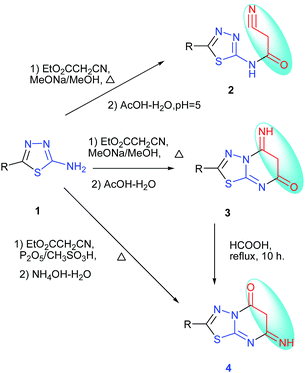 | ||
| Scheme 2 The synthesis of 6,7-dihydro-7-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one. | ||
In order to obtain new 6,7-dihydro-7-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives, the reaction of 2-amino-5-substituted-1,3,4-thiadiazoles, 2a–o and ethyl cyanoacetate under the presence of phosphorus pentoxide and formic acid was utilized. The phosphorus pentoxide or formic acid was utilized respectively to catalyze the reaction of 2-amino-5-substituted-1,3,4-thiadiazoles 2a–o and ethyl cyanoacetate, but phosphorus pentoxide and formic acid have not been utilized together in the reaction. Here, we obtained the synthesis of a new series of 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives 3a–o. The synthesis pathway is shown in Table 1.
| Entry | Substrate | R | Yielda (%) |
|---|---|---|---|
| a Isolated yield. | |||
| 1 | 3a | 2-Ethoxyphenyl | 90 |
| 2 | 3b | 4-Methylphenyl | 75 |
| 3 | 3c | 3-Methoxyphenyl | 78.5 |
| 4 | 3d | 4-Methoxyphenyl | 93 |
| 5 | 3e | Phenyl | 30 |
| 6 | 3f | 4-Chlorophenyl | 48 |
| 7 | 3g | 4-Bromophenyl | 45 |
| 8 | 3h | Furan-2-yl | 52.5 |
| 9 | 3i | 2-Chlorophenyl | 69.5 |
| 10 | 3j | 3-Methylphenyl | 82 |
| 11 | 3k | 2-Bromophenyl | 56 |
| 12 | 3l | 2-Fluorophenyl | 62 |
| 13 | 3m | Benzyl | 67 |
| 14 | 3n | 2-Methoxyphenyl | 85 |
| 15 | 3o | 2-Methylphenyl | 76 |
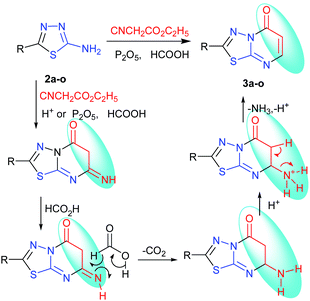
The tandem reaction of diversity-oriented synthesis was carried out by a method of typical three-step synthesis to a one-pot process in the presence of phosphorus pentoxide–formic acid. However, the target product was given by formic acid which was thought to be the reductant of Eschweiler–Clarke and Leuckart–Wallach reaction conditions.20,21 The synthesis of a new series of 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives 3a–o was a combination of [3 + 3] cycloaddition, reduction, deamination reactions with 30–93% yield, which is the tandem reaction. The reaction was a combination of many types and steps of reactions in a one-pot reaction, 7-imino was obtained by Tsuji modes,16 and was eliminated by tandem reaction of reduction and deamination when methanesulfonic acid–phosphorus pentoxide was changed to formic acid–phosphorus pentoxide as the catalytic agent. Compounds 2a–o were synthesized by the method reported in the literature.18,22–32 The mechanism of the synthesis is shown in Scheme 3.
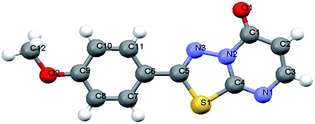
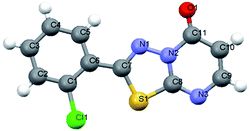
From the above discussion, the synthesis of target product was explored by 2-amino-5-(4-methylphenyl)-1,3,4-thiadiazole, ethyl cyanoacetate, phosphorus pentoxide and concentrated H2SO4. The reaction was carried out at 100–105 °C for 12 h, monitored by TLC, which didn't give relevant target product. Then, the synthesis of the target product was probed by the addition of 2-amino-5-(2-ethoxyphenyl)-1,3,4-thiadiazole, ethyl cyanoacetate, phosphorus pentoxide and glacial acetic acid, and this also didn't give the target product. When we chose only phosphorus pentoxide–formic acid as condensation reagent at the same conditions, the target product (3d) was obtained with a yield of 93%. Phosphorus pentoxide–formic acid was an efficient condensation regent for the synthesis of 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives 3a–o. The synthesis reaction didn't give target product by 2-amino-5-substituted-1,3,4-thiadiazole, ethyl cyanoacetate, phosphorus pentoxide and glacial acetic acid (or concentrated H2SO4 or methanesulfonic acid), but the target product was given by formic acid which is the reducing reagent as Leuckart–Wallach, Eschweiler–Clarke reaction conditions. The tandem reaction of 2-amino-5-substituted-1,3,4-thiadiazole, ethyl cyanoacetate and methanesulfonic acid–phosphorus pentoxide gave 6,7-dihydro-7-imino-2-substituted-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one.16 Then the imino group of 7-position was reduced and converted to amino group by formic acid.20,33 After elimination of ammonia, the target product was given under acid alike Fischer indole synthesis.34 Described herein is our approach, which reduces the typical three-step synthesis to a one-pot process. The mechanism of the reaction is shown in Scheme 3. The molecular structure of the product 3d and 3i was established by X-ray diffraction (Fig. 1, Fig. 3). The supramolecular structure of compound 3d and 3i is shown in Fig. 2 and Fig. 4.
As an extension of the synthetic methodology, some 4H-pyrido[1,2-a]pyrimidin-4-one 4a–e was synthesized. The synthesis pathway is shown in Table 2.
Conclusions
In conclusion, a novel and efficient one-pot method has been developed for the synthesis of 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivative by the tandem reaction of [3 + 3] cycloaddition, reduction, deamination reactions under the presence of formic acid-phosphorus pentoxide as the catalytic agent. The fused heterocyclic compounds 2-substituted-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-ones was synthesized by the diversity-oriented catalysis.Notes and references
- B. W. Clare and C. T. Supuran, Eur. J. Med. Chem., 2000, 35(9), 859–865 CrossRef CAS PubMed.
- B. H. Lee, M. F. Clothier, F. E. Dutton, G. A. Conder and S. S. Johnson, Bioorg. Med. Chem. Lett., 1998, 8, 3317–3320 CrossRef CAS PubMed.
- S. S. Shuknrow, M. A. Knkhanov, I. M. Nasyrov, K. S. Iakharov and R. A. Karakhananov, Russ. Chem. Bull., 1993, 42, 1871–1874 CrossRef.
- F. Russo, M. Santagati, A. Santagati, A. Caraso, S. Trombatere and M. A. Roxas, Farmaco, Ed. Sci., 1983, 38, 762–774 CAS.
- I. F. Russo, M. Santagati, A. Stagati and G. Blandino, Farmaco, Ed. Sci., 1981, 36, 983–990 Search PubMed.
- M. Sniko, E. Taniguchi, K. Maekawa and M. Eto, Agric. Biol. Chem., 1980, 44, 1923–1927 CrossRef.
- M. Suiko, E. Taniguchi, K. Maekawa and M. Eto, Agric. Biol. Chem., 1979, 43, 741–746 CrossRef CAS.
- R. Blue and B. S. Coller, WO 2008057601, 2008.
- K.-C. Liu, S.-Y. Chem, T.-M. Tao and L.-C. Lee, Arch. Pharm., 1979, 312, 619–622 CrossRef CAS PubMed.
- M. Suiko, K. Maekawa and M. Eto, Agric. Biol. Chem., 1977, 41, 2047–2053 CrossRef CAS.
- H. Paul and A. Sitte, Monatsh. Chem., 1971, 102, 550–557 CrossRef CAS.
- A. Shafiee and L. Lalezari, J. Heterocycl. Chem., 1975, 12, 675–681 CrossRef CAS.
- R. F. Lauer and G. Zencheff, J. Heterocycl. Chem., 1976, 13, 291–293 CrossRef CAS.
- G. Kornis, P. J. Marks and G. G. Chidester, J. Org. Chem., 1980, 45, 4860–4863 CrossRef CAS.
- (a) F. Russo, A. Santagati and M. Stantagati, J. Heterocycl. Chem., 1985, 22, 297–299 CrossRef CAS; (b) T. Tsuji, J. Heterocycl. Chem., 1991, 28, 489–492 CrossRef CAS.
- (a) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314–325 CrossRef CAS PubMed; (b) H. R. Dong, W. J. Dong, R. S. Li, Y. M. Hu, H. S. Dong and Z. X. Xie, Green Chem., 2014, 16, 3454–3457 RSC; (c) S. X. Zhai, H. R. Dong, Z. B. Chen, Y. M. Hu and H. S. Dong, Tetrahedron, 2014, 70, 8405–8412 CrossRef CAS.
- B. Busch, A. T. Johnson, R. Martin, R. Mohan and W. C. Stevens, WO 2005072731, 2005.
- (a) A. Santagati, M. Santagati, F. Russo and G. Ronsisvalle, J. Heterocycl. Chem., 1988, 25, 949–953 CrossRef CAS; (b) P. Mullick, S. A. Khan, S. Verma and O. Alam, Bull. Korean Chem. Soc., 2010, 31(8), 2345–2350 CrossRef CAS; (c) Y. Yu, Asian J. Chem., 2007, 19(4), 3141–3144 CAS.
- K. Takenaka and T. Tsuji, J. Heterocycl. Chem., 1996, 33, 1367–1370 CrossRef CAS.
- (a) M. Kitamura, D. Lee, S. Hayashi, S. Tanaka and M. Yoshimura, J. Org. Chem., 2002, 67, 8685–8687 CrossRef CAS PubMed; (b) A. Côté and A. B. Charette, J. Org. Chem., 2005, 70, 10864–10867 CrossRef PubMed.
- (a) G. Bobowski, J. Org. Chem., 1985, 50, 929–931 CrossRef CAS; (b) L. B. Julia and P. O'Brien, J. Org. Chem., 2008, 73, 6452–6454 CrossRef PubMed.
- G. Tu, S. Li, H. Huang, G. Li, F. Xiong, X. Mai, H. Zhu, B. Kuang and W. Xua, Bioorg. Med. Chem., 2008, 16, 6663–6668 CrossRef CAS PubMed.
- D. R. Guda, H. M. Cho and M. E. Lee, RSC Adv., 2013, 3, 6813–6816 RSC.
- G. Kolav, V. Hegde, I. A. Khazi and P. Gadad, Bioorg. Med. Chem., 2006, 14, 3069–3080 CrossRef PubMed.
- (a) S. Ferrari, F. Morandi, D. Motiejunas, E. Nerini, S. Henrich, R. Luciani, A. Venturelli, S. Lazzari, S. Calò, S. Gupta, V. Hannaert, P. A. M. Michels, R. C. Wade and M. P. Costi, J. Med. Chem., 2011, 54, 211–221 CrossRef CAS PubMed; (b) T. Sakamoto, M. Moriya, H. Tsuge, T. Takahashi, Y. Haga, K. Nonoshita, O. Okamoto, H. Takahashi, A. Sakuraba, T. Hirohashi, T. Shibata, T. Kanno, J. Ito, H. Iwaasa, A. Gomori, A. Ishihara, T. Fukuroda, A. Kanatani and T. Fukami, Bioorg. Med. Chem., 2009, 17, 5015–5026 CrossRef CAS PubMed; (c) D. C. Pryde, G. N. Maw, S. Planken, M. Y. Platts, V. Sanderson, M. Corless, A. Stobie, C. G. Barber, R. Russell, L. Foster, L. Barker, C. Wayman, P. Van Der Graaf, P. Stacey, D. Morren, C. Kohl, K. Beaumont, S. Coggon and M. Tute, J. Med. Chem., 2006, 49, 4409–4424 CrossRef CAS PubMed.
- F. Z. Smaine, F. Pacchiano, M. Rami, V. B. Montero, D. Vullo, A. Scozzafava, J. Y. Winum and C. T. Supuran, Bioorg. Med. Chem. Lett., 2008, 18, 6332–6335 CrossRef CAS PubMed.
- X.-H. Liu, Y.-X. Shi, Y. Ma, C.-Y. Zhang, W.-L. Dong, L. Pan, B.-L. Wang, B.-J. Li and Z.-M. Li, Eur. J. Med. Chem., 2009, 44, 2782–2786 CrossRef CAS PubMed.
- J. K. Thomas and M. C. Silvio, J. Heterocycl. Chem., 1980, 17, 607–608 CrossRef.
- S.-H. Li, G. Li, H.-M. Huang, F. Xiong, C.-M. Liu and G.-G. Tu, Arch. Pharmacal Res., 2008, 31, 1231–1239 CrossRef CAS PubMed.
- K. Zheng, J. He and J. Zhang, Chin. Chem. Lett., 2008, 19, 1281–1284 CrossRef CAS.
- R. Rani and J. K. Makrandi, Indian J. Heterocycl. Chem., 2008, 18, 121–124 CAS.
- S. G. Alegaon and K. R. Alagawadi, Med. Chem. Res., 2012, 21, 816–824 CrossRef CAS.
- (a) A. Lukasiewiez, Tetrahedron, 1963, 19, 1789–1799 CrossRef CAS; (b) R. Kadyrov and T. H. Riermeier, Angew. Chem., Int. Ed., 2003, 42, 5472–5474 CrossRef CAS PubMed; (c) R. Bianchini, C. Forte, G. Musumarra, C. Pinzino and C. Sergi, Tetrahedron, 1997, 53, 6907–6916 CrossRef CAS.
- (a) D. L. Hughes and D. Zhao, J. Org. Chem., 1993, 58, 228–233 CrossRef CAS; (b) G. Bhattacharya, T.-L. Su, C.-M. Chia and K.-T. Chen, J. Org. Chem., 2001, 66, 426–432 CrossRef CAS PubMed.
- 3d, C12H9N3O2S, crystal size 0.35 × 0.32 × 0.29 mm3, space group C2/c (monoclinic), a = 14.1942 (13), b = 13.4679 (13), c = 12.8940 (12) Å, β = 112.384 (7)°, V = 2279 (4) Å3, Z = 8, ρcalcd = 1.511 g cm−3, μ (MoKα) = 0.281 mm−1. Data were measured on a Bruker SMART APEX diffractometer (MoKα radiation, λ = 0.71073 Å) at 296 K, and the structure was solved by direct methods. Of 5973 reflections collected, 2116 were unique reflections (Rint = 0.0336); data/restraints/parameters 2116/0/165; final R indices [I > 2σ(I)]: R1 = 0.0422, wR2 = 0.0982; R indices (all data): R1 = 0.0677, wR2 = 0.1099. ESI.†.
- 3i, C11H6ClN3OS, crystal size 0.38 × 0.35 × 0.31 mm3, space group P2(1)/n (monoclinic), a = 10.838 (4), b = 4.9244 (19), c = 20.011 (7) Å, β = 98.505 (6)°, V = 1056 (7) Å3, Z = 4, ρcalcd = 1.658 g cm−3, μ (MoKα) = 0.542 mm−1. Data were measured on a Bruker SMART APEX diffractometer (MoKα radiation, λ = 0.71073 Å) at 296 K, and the structure was solved by direct methods. Of 5348 reflections collected, 2061 were unique reflections (Rint = 0.0237); data/restraints/parameters 2061/0/154; final R indices [I > 2σ(I)]: R1 = 0.0330, wR2 = 0.0846; R indices (all data): R1 = 0.0397, wR2 = 0.0902. ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 779780 and 779674. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra02714k |
| This journal is © The Royal Society of Chemistry 2014 |