Morphology controlling of calcium carbonate by self-assembled surfactant micelles on PET substrate†
Zhenyou Lia,
Li Xinga,
Junhui Xiang*a,
Xiaohong Liang*b,
Chunlin Zhaoa,
Huazheng Saia and
Fei Lia
aCollege of Materials Science and Opto-Electronic Technology, University of Chinese Academy of Sciences, Yuquan Road 19A, Beijing, 100049 China. E-mail: xiangjh@ucas.ac.cn; Fax: +86 10 88256532; Tel: +86 10 88256532
bCollege of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China. E-mail: liangxiaohong@tyut.edu.cn
First published on 23rd June 2014
Abstract
Spherical and hexagonal CaCO3 with hierarchical structures were prepared on surfactant-modified PET substrate between the interfaces of saturated Ca(OH)2 solution and n-hexane. The results show that CaCO3 synthesis follows the same nanoparticle-mediated self-organization process but the morphology of the products strongly depends on the properties of surfactant modification. The synthetic procedure offers several important characteristics for CaCO3 superstructure fabrication. Surfactants in this experimental system act not only as templates, but also as regulators for the fabrication of superstructures. This biomimetic system will be promising in synthesizing other functional nanoparticles or nanodevices.
Introduction
Hierarchical structure of biomaterials that exhibit uniform morphology and outstanding mechanical properties are universal in living organisms.1 One secret of biomaterials' superior properties is their organic–inorganic hybrid structure whereby the precise arrangement of building blocks is achieved over several length scales.2 Organic components, biomacromolecules and organic matrices can regulate crystal nucleation and growth, modulate crystal shape and size, and control the organization of building blocks into complex structures, and have been an active area of research during the past few years.3–5Calcium carbonate is one of the most abundant biomaterials in nature, mainly as exoskeleton in shells and cell walls or as mechanical support in spicules and spines,1 exhibiting different structures and properties to carry out specific functions. Its crystal structure, size, and shape are major concerns in crystallization and have recently received a significant amount of attention. Several efforts have been made to mimic the biomineralization process to prepare CaCO3 crystals with defined characteristics.5–7 These biological strategies can be organized into two categories: (1) mineralization in insoluble matrices such as polymeric scaffolds,8 biopolymer gels9–11 or self-assembled monolayers (SAMs),12–14 and (2) crystal growth with the help of soluble additives such as polymers,15 copolymers,16 or surfactants.17 The latter soluble molecules are believed to participate in both nucleation and growth processes by either binding selectively to certain families of crystal planes18,19 or interacting with charged ions to stabilize unstable phases,20 which is considered to be an effective and versatile method. Therefore, most researchers emphasize finding new molecules with regulating effect and investigating how the regulating effect works. For instance, calcium carbonate crystal with spiky dumbbell-like superstructure was synthesized in the presence of casein, a typical phosphoprotein.21 Wei et al. used a non-ionic peptide-type block copolymer as a structure-directing agent to produce calcite with stepped (104) face at the air–solution interface.16 In most of these works, soluble additives are present in the form of molecules. However, these amphipathic molecules are easy to assemble in different shapes of micelles. In addition, the micelle itself is a good template. Thus, these micelles are better candidates to regulate the shape of CaCO3 crystals. However, very few studies have used soluble additive micelles to direct the growth of the crystals.22,23
As most crystallizations occur in solution, the biggest problem with this issue is the dynamic nature of micelles in solution. The shape of micelles in solution is strongly affected by the solution environment. As time varies, solution properties of the solution, where the crystallization reaction occurs, also change. Thus, the shape of the micelles is different; the micelles may even disappear. Compared with bulk solution, solid–liquid interface provides a unique environment for additive molecules assembly. The assembled patterns are mainly determined by the substrate and additive properties (such as hydrophobic property and surface charge density), which are always different from those in free solution.24–28 This makes it possible to control the morphology of crystals by anchoring the self-assembled micelles on solid substrate.29–31
In the previous study,14 we revealed the basic rules of forming superstructure materials via a nanoparticle-mediated self-organization process. We found that the synergetic effects between organic–inorganic interfaces and self-assembled monolayers (SAMs) are the essential factors for the control of hierarchical structures. SAMs can induce the direction and arrangement of the building blocks. Indeed, the regulation of the substrate will be enhanced if the surfactant micelles are further modified on SAMs. The stronger effect of surfactant micelle-modified substrate relies not only on the larger amount of functional groups, but also on the template effect of the micelles.
Therefore, in this work, the regulation effects of surfactant micelle-modified poly (ethylene terephthalate) (PET) substrate on CaCO3 morphology were studied. In addition, spherical and hexagonal CaCO3 hierarchical structures were produced, respectively, when cetyl betaine and Triton X-100 were induced. Herein, the organic–inorganic interface provides an independent synthetic environment from bulk solution. The surfactants act not only as structure-directing agents inducing the orientation of the particles, but also as templates guiding them to their final morphology. In both the conditions, the formation of CaCO3 hierarchical structures follows the same nanoparticle-mediated self-organization process, which provides us with a facile way to produce hierarchical nanomaterials with defined structures.
Experimental
Materials
Calcium hydroxide, ammonium bicarbonate, n-hexane, ethanol, acetone, toluene and p-octyl polyethylene glycol phenyl ether (Triton X-100) were purchased from Beijing Chemical Reagents Company. Aminopropyl triethoxysilane (APTES) was purchased from Alfa Aesar. Octadecyltrichlorosilane (OTS) was purchased from Acros Organics. Cetyl betaine was purchased from Chem Service Company. All the chemicals were of analytical grade and used as received without further purification. All the glassware was cleaned by sonication in water for 5 min, rinsed with distilled water, and finally dried at 105 °C.Fabrication of hydrophobic and hydrophilic surface of the polymer substrate
By assembling octadecyltrichlorosilane (OTS) on PET substrate, the hydrophobic surface of PET substrate was fabricated according to the reported methods.32–34 In a typical experiment, APTES was dissolved in acetone (1 vol%) and aged for 240 h. PET substrate was ultrasonically cleaned for 5 min successively in distilled water, ethanol, and acetone and then dried at 105 °C for 5 min. The dried substrate was immersed in an acetone solution of APTES for 5 min and baked at 105 °C for 5 min to form a buffer layer. After that, the substrate grafted by buffer layers was immersed in the OTS solutions (1 vol% in toluene) under a nitrogen atmosphere for 2 min and then rinsed with anhydrous toluene and baked at 105 °C for 5 min to form the hydrophobic surface of PET substrate. The hydrophilic surface of PET substrate was obtained by irradiating the hydrophobic PET substrate with ultraviolet (UV) light. The UV-irradiated surface became hydrophilic because of the formation of hydroxyl groups.Self-assembly of surfactant on the modified PET substrate
PET substrates with hydrophobic/hydrophilic surface were immersed in the saturated solution of surfactant for 30 min at 80 °C, slightly rinsed with deionized water, and then dried at 105 °C. In this manner, surfactant molecules assembled at the solid–liquid interface. Here, cetyl betaine and Triton X-100 were chosen for the study.CaCO3 growth on the surfactant-assembled substrate
Room-temperature mineralization of CaCO3 on the PET substrate was performed at the organic–inorganic liquid interface by a slow gas-diffusion procedure. The organic phase was n-hexane while the inorganic phase was Ca(OH)2 saturated solution with 500 μL saturated surfactant solution. The nucleation and growth of CaCO3 was induced by carbon dioxide from the decomposition of NH4HCO3 diffusing into the system (Scheme 1). After a period of time, the substrates covered with CaCO3 were removed, and the overgrown specimens were slightly rinsed with deionized water. Then, the as-grown CaCO3 on the substrate was dried in air for further characterization.Characterization of CaCO3 deposited on substrate
The processes of silane and surfactant self-assembly were characterized by water contact angle through JC2000C1 (POWEREACH). Small pieces of the as-prepared CaCO3 on modified PET substrates were coated with gold for scanning electron microscopy (SEM) measurements on a JMS-6301F microscope. An X-ray diffractometer (XRD, MSAL XD-2 powder X-ray diffractometer) operating at 30 kV and a current of 30 mA with Cu Kα radiation, λ = 1.54056 Å, was also used for characterizing the samples. Infrared spectroscopic analysis was performed in the transmission mode (FT-IR) using a VERTEX 70 with scanning from 4000 to 500 cm−1.Results and discussion
Surfactant assembly on PET substrate
In this research, zwitterionic surfactant cetyl betaine and nonionic surfactant Triton X-100 were chosen to modify both the hydrophilic and hydrophobic substrates. The surface properties of substrates were characterized with a water contact angle device, and the results are shown in Fig. 1. Contact angles of cetyl betaine micelle-modified hydrophilic substrate and Triton X-100 are 5° and 7°, respectively. Compared with the hydrophilic surface of blank PET, the contact angle became smaller after assembling either cetyl betaine or Triton X-100, whereas for the hydrophobic substrates, the contact angle did not change after surfactant assembly, remaining at 105°.The drop of the contact angle of the modified hydrophilic substrate results from a change of functional groups on the surface, indicating the formation of surfactant micelles on the substrate. In the saturated aqueous solutions, whose concentrations were above the critical micelle concentration (CMC), surfactants existed almost in the form of micelles with head groups outward to form hydrophilic membrane. Because the head groups of surfactant have a strong affinity toward hydrophilic substrate, micelles could be easily assembled onto the substrate when the substrate terminated with hydroxyl groups was immersed on the saturated aqueous solutions of surfactants.35–37 Fig. 2 shows the SEM images of PET substrate before and after modification. Before modification, both hydrophilic and hydrophobic substrates have a smooth surface. Bright spots on the substrates are the sputtered Au nanoparticles. After modification, cetyl betaine micelles and Triton X-100 micelles are formed only on the hydrophilic substrates. Fig. 2b shows that cetyl betaine micelle-modified substrates are spherical while Triton X-100 micelles have spindle morphology, as shown in Fig. 2c. What should be pointed out here is that because the drying process will affect micelle structure, SEM images may not reflect the structures formed in situ. Although SEM data are speculative, these surfactant aggregates on the substrates also suggested a successful modification. Given the experimental results mentioned above, we proposed a possible modification process with detailed description, which is shown in Fig. 3. After the assembly of cetyl betaine micelles, the terminal groups of the substrate become –COO− while the surface groups of the blank PET are hydroxyl. The difference between the deprotonated carboxyl groups against hydroxyl groups benefits the improvement of hydrophilicity and the water contact angle becomes smaller. In the case of Triton X-100, the surface groups are still hydroxyl. However, as assemblies of micelles, more hydroxyl groups are formed on the surface of the substrate. Thus, the modified substrate has denser hydroxyl groups than that of the blank PET, becoming a more hydrophilic substrate with the water contact angle reduces.
 | ||
| Fig. 2 SEM images of hydrophilic PET (a), treated with cetyl betaine (b) and Triton X-100 (c). SEM images of hydrophobic PET (d), treated with cetyl betaine (e) and Triton X-100 (f). | ||
 | ||
| Fig. 3 Formation of surfactant micelles on hydrophilic substrates. (a) Molecular formula of cetyl betaine; (b) molecular formula of Triton X-100. | ||
The final state of surfactant aggregates was a balance of interactions between surfactant, solvent, and a solid substrate on the aggregation. To make it simple, we studied only net neutral surfactants. (For zwitterionic surfactant cetyl betaine, the negative charged carboxyl was neutralized by quaternary ammonium cation.) The strong monopolar charge–charge interactions that occur for anionic or cationic surfactants were minimized. Under this condition, the surfactant aggregates were more strongly affected by solution conditions, substrate property and neighboring aggregates.30 In this research, the authors chose saturated aqueous solutions for surfactant assembly, in which the concentration was higher than that of bulk CMC. Therefore, most of the surfactant in solution formed spherical aggregates, and the surfactant micelles' morphology was no longer a function of concentration. Herein, the difference between the assembled aggregates of cetyl betaine and Triton X-100 contributed to their different head groups. Thus, it is reasonable to consider the structural change in the micelles from solution to substrate because of the replacement of water–headgroup interactions with surfactant–solid interactions. There is a correlation between surfactant curvature and the affinity of the head group for the solid substrate. On one hand, a high density of surface sites that can form strong bonds between head groups and substrate will lead to a strong perturbation of micelle structure. Thus, a less curved micelle will form as the density of available strong-bonding sites increases. For Triton X-100, its head groups are hydroxyl, which can hydrogen-bond with the hydroxyl groups on the substrate. This strong interaction may be the main contributor to the structural change after assembling. Therefore, the curvature becomes smaller, forming spindle-shaped surfactant micelles on the substrate. On the other hand, if the substrate forms low energy bonds with the surfactant head groups, it is reasonable to have slightly perturbed micelles attached to the solid. The head groups of cetyl betaine are carboxyl groups and quaternary ammonium cations, which can interact with the substrate by hydrogen bonds. However, the hydrogen bonds between the cetyl betaine and the substrate are weaker than those of the Triton X-100. This relatively weak interaction would not change the shape of the micelles after assembling; they would remain spherical. Furthermore, the repelling interaction between carboxyl groups causes the distances between the cetyl betaine aggregate units to be larger than that of Triton X-100 (Fig. 2b–c).
While for the hydrophobic substrates, the contact angle had nearly no change after immersing into the surfactant-saturated aqueous solutions (Fig. 1d–f). There was an alkyl chain layer on the surface of the OTS-modified hydrophobic substrate; thus, the solid substrate could not form hydrogen bonds with the surfactant head group. In this situation, a large free-energy reduction of the system was made possible by covering up the solid substrate with surfactant hydrocarbon tails. Therefore, free surfactant monomers or hemicylinder micelles could be assembled onto the substrates by hydrophobic interactions.37 However, the results showed that the water contact angle of hydrophobic substrate was not changed. SEM images of this kind of substrate before and after treating with surfactant solutions (Fig. 1d–f) had also shown that the assembly of surfactants did not occur. This indicated that there were few free surfactant monomers or hemicylinder micelles in the saturated solutions, and the reduction of free energy was not sufficient to perturb the structure of spherical micelle in the bulk solution.
It is worth noting that surfactant micelles can only self-assemble on hydrophilic substrate. As the hydrophilic substrate can be obtained by irradiating the hydrophobic PET substrates with UV light, if we expose only one side of the substrate to UV light, the exposed side becomes hydrophilic while the other side remains hydrophobic. When the substrate was immersed into surfactant solution, the micelles assembled only on the hydrophilic side, and the other side remained hydrophobic. The amphipathy of the substrate allows it to float between organic–inorganic interfaces, which make the following study possible.
Morphology of CaCO3 on substrates
Cetyl betaine and Triton X-100 surfactant micelle-modified substrates were put at the organic–inorganic interface separately with the hydrophilic side towards the aqueous phase. After 48 h of reaction, different shapes of CaCO3 crystals were grown on these substrates. When cetyl betaine micelle-modified substrates were induced in the reaction system, spherical particles of about 30 μm diameter were fabricated (Fig. 4a). The inset picture of Fig. 4a is the low-magnification image of CaCO3 samples, which shows that the samples are composed of a large quantity of well-dispersed spherical particles with uniform diameter. The surface appearance of sphere was clearly visible in Fig. 4c, showing triangular pyramidal morphology radiating from the centre of the crystals. These pyramids were 1 μm in height with similar shape and smooth surfaces. The typical image of the cracked sample in Fig. 4d shows that the CaCO3 spheres are composed of several small sticks. In addition, we should also notice the hole (indicated by the black arrow) in the centre of the cracked sphere, suggesting a core in the spherical crystal. The size of the hole is about 1–2 μm, which is just the same as the size of the cetyl betaine micelle. These results reveal that the micelles have a template role in the growing process. More detailed information regarding the spherical structure can be further provided by XRD analyses. In Fig. 4b, the XRD spectrum clearly indicates that the as-prepared products are composed of a calcite phase with the characteristic (104), (110), (113), (202) and (116) planes (JCPDS card no. 05-0586). The two broad peaks observed at 47.2° and 54.1° were consistent with the XRD pattern of blank PET shown in Fig. S1.† The random orientations of the crystals suggest that cetyl betaine molecules have relatively weak interaction with the planes of calcite crystals. Thus, adsorption on specific planes did not occur. The controlled experiment was conducted when cetyl betaine micelle-modified substrate was removed from the system. The obtained products showed a rhombohedral calcite phase with an average size of over 50 μm (Fig. S2†), revealing that the surfactant has an important influence on the morphology of CaCO3. | ||
| Fig. 4 (a, c and d) Typical SEM images and (b) corresponding XRD pattern of CaCO3, which were fabricated on cetyl betaine-assembled hydrophilic substrate between n-hexane–aqueous interfaces. Inset in (a) is low-magnification image of specimen presented in Fig. 2a. | ||
The introduction of Triton X-100 micelles onto the substrate resulted in the formation of hexagonal CaCO3 crystals. In Fig. 5a, the hexagon was 4 μm in length and 3 μm in width. The inset picture of Fig. 5a shows a large number of hexagonal CaCO3 with high nucleation densities and narrow-spread particle size on the substrate. The rough surface of the hexagonal crystals can be seen at high magnification in Fig. 5c and d. The corresponding XRD spectrum in Fig. 5b indicates that the as-synthesized products were composed of a calcite phase with the characteristic (104) and (202) planes. Similarly, the two broad peaks at 47.2° and 54.1° belonged to blank PET (Fig. S1†). Without the Triton X-100 micelles, CaCO3 crystals grow in random directions, resulting in several other planes in the XRD curve.14 This implies that Triton X-100 molecules have strong interaction with the (104) and (202) planes. When absorbing on the planes, they can inhibit the crystals from growing vertical in direction. Thus, these planes remained while others disappeared. CaCO3 mineralizing without Triton X-100 results in the formation of large rhombohedral calcite crystals with an average size over 50 μm (Fig. S2†), revealing that the surfactant has a significant influence on crystalline CaCO3.
 | ||
| Fig. 5 (a, c and d) Typical SEM images and (b) corresponding XRD pattern of CaCO3, which were fabricated on Triton X-100-assembled hydrophilic substrate between n-hexane–aqueous interfaces. Inset in (a) is low-magnification image of specimen presented in Fig. 3a. | ||
Both the spherical and hexagonal products are different from those obtained from the organic–inorganic interface. In addition, CaCO3 crystals grow evenly on the hydrophilic side of the substrate, while on the hydrophobic side, we can only find very few rhombohedral crystals at the edge of the substrate. All this evidence shows that surfactant micelle-modified substrates play a key role in modulating the morphology of the crystals.
Roles of surfactant on CaCO3 morphology
To further demonstrate the important role of the surfactant, the morphological development of CaCO3 at different crystallization times was examined. Representative SEM images of the products obtained at 2 h, 12 h, 24 h and 48 h are presented in Fig. 6 and 7.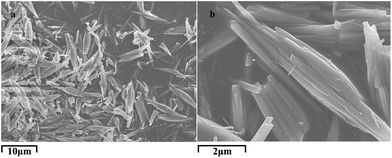 | ||
| Fig. 7 SEM images of rod-like CaCO3 crystals obtained from substrate-free system. The surfactant in the inorganic phase is cetyl betaine. | ||
Fig. 6 shows the entire growing process when cetyl betaine was induced in the system. The products of Fig. 6a were collected directly from the organic–inorganic interfaces (not on the substrate) after 2 h of reaction. It is clear that the first species to form are amorphous calcium carbonate sphere particles of about 50 nm in diameter. This is in good agreement with the previous works.14,38,39 When the reaction time was prolonged to 12 h, the as-prepared products were rod-like crystals of 20 μm in length and 5 μm in diameter. When crystallized for 24 h, sheaf-like CaCO3 hierarchical structures appeared. Finally, spherical CaCO3 crystals were formed. The entire process of crystal growth on substrate is similar to flowers in bloom. In addition, the size of the crystals did not change from 12 h to 48 h, which implies that the further growing process is just a continual assembly of the building blocks. This result also reminds us of the earlier findings in Fig. 4d that the CaCO3 spheres are composed of small sticks. To obtain more evidence regarding the formation of these building blocks, the morphology of CaCO3 grown in the same but substrate-free system was examined. In this system, modulating effect of functional substrates was removed but the surfactant in the aqueous phase still regulated crystal growth. The corresponding images in Fig. 7 show the products have similar size and shape to the building blocks. This is direct evidence that the CaCO3 sticks, which finally assembled into spherical CaCO3 hierarchical structure were formed with the help of the surfactant in the aqueous phase.
In the case of Triton X-100-modified substrate, the growth of the hexagonal CaCO3 followed a similar process. Fig. 8a shows the microstructure of CaCO3 obtained from the organic–inorganic interface (not on the substrate) after 2 h of crystallization. It can be seen that the products consisted of 30 nm nanoparticles, revealing that the early stage of the hexagonal products were also amorphous calcium carbonate. With time, spindle-shaped and hexagonal CaCO3 were fabricated on the substrate. Comparing Fig. 8b–d, we can see a smooth surface is gradually forming at the end of the spindle, which reveals a ripening process in the crystal growth. In order to get more detail about the formation mechanism, we construct a same but substrate-free system. After 24 h of reaction, calcite crystals with layered structure have been found as shown in Fig. 9. It is worth noting that products grown on the Triton X-100 micelle-modified substrate (Fig. 5d) have similar structures, which means surfactant molecules in solution play a significant role in forming the structure and morphology of the hexagonal CaCO3. And, this layered structure comes from the specific adsorption of Triton X-100 on (104) and (202) planes (see the XRD pattern in Fig. 5b).4,16
 | ||
| Fig. 9 SEM images of rod-like CaCO3 crystals obtained from substrate-free system. The surfactant in the inorganic phase is Triton X-100. | ||
In addition, in both conditions, the density of the crystals on the substrates is high at the edges while low in the middle. This phenomenon reveals CaCO3 growth begins from the edges of substrates and indicates a self-organization process on the substrates.
Based on the experiment results mentioned above, it could be suggested that CaCO3 synthesis follows the same nanoparticle-mediated self-organization processes. However, the final morphology of products depends on the shapes of the micelles on the substrate. Scheme 2 shows the possible formation of CaCO3 crystals. At the beginning of CaCO3 growth, carbon dioxide from the decomposition of ammonium bicarbonate reacted with H2O to enrich the carbonate ions and then induce heterogeneous precipitation to form nanoparticles between the organic–inorganic interfaces. In this process, the terminal groups of the surfactant in the aqueous solution may affect the particles formation. For cetyl betaine, the deprotonated –COO− groups have the ability to bind calcium ions by electrostatic interaction. After that, these nanoparticles are likely to transfer to the substrate, interacting with the surface groups of the cetyl betaine micelles through electrostatic interactions, hydrogen bonds or van der Waals forces. At the same time, these nanoparticles with random crystallographic orientations and high internal energy are growing gradually to fuse with each other and tending to lower the energy by arranging orderly and assembling into CaCO3 sticks (Scheme 2a-1). Consequently, these sticks may rotate to achieve the same orientation with the driving force coming from specific absorption of the surfactant and the removing of surface energy which is shown in Scheme 2a-2.40 Meanwhile, some other CaCO3 nanoparticles attach to the sticks by Brownian motion and form more sticks in the same manner. With an increasing of crystallization time, these building blocks (the sticks) begin to aggregate at both ends of the CaCO3 nanorods. (Scheme 2a-3) Finally, CaCO3 hierarchical structures with unusual morphologies are formed by continually attaching sticks or nanoparticles. (Scheme 2a-4) When cetyl betaine is replaced by a nonionic surfactant (Triton X-100), CaCO3 growth suffers the same process. CaCO3 nanoparticles formed at the interfaces will transfer to the substrate and absorb on the micelles. (Scheme 2b-1) These nanoparticles tend to fuse with each other and crystallize into hexagonal calcite crystals (Scheme 2b-2–b-3). At this time, surfactant molecules (Triton X-100) in the saturated Ca(OH)2 solution may have strong interaction with some specific planes of CaCO3 and are absorbed on these planes. Thus, crystal growth along this direction will be constrained. When other nanoparticles are absorbed on the hexagonal crystals, they can only grow vertical to this direction. Finally, hexagonal CaCO3 crystals with layered structure are formed after hours of reaction.
 | ||
| Scheme 2 Possible formation process of CaCO3 crystals on cetyl betaine micelle-modified substrate (a-1–a-4) and Triton X-100 micelle-modified substrate (b-1–b-4). | ||
Most of the time, the balance between crystal growth and mass transport is an important factor in controlling the morphology of crystals. However, herein, surfactant micelles assembled on substrate play the leading role in influencing final morphologies. In addition, the size of CaCO3 crystals is also affected by micelles. The cetyl betaine micelle (Fig. 1b) has a bigger size than the Triton X-100 micelle (Fig. 1c). Then, after crystallization, the spherical CaCO3 flowers (Fig. 4a) are therefore bigger than the hexagonal crystals (Fig. 5a). Moreover, the surfactant micelles in the biomimetic mineralization system are acting not only as a template, but also as an inhibitor, preventing CaCO3 crystallizing. In the FTIR spectra (Fig. 10), the presence of the peak at 1082 cm−1 is attributed to symmetric stretch of carbonate ion in plane (ν1), and the split peaks at around 1387 could be seen as its asymmetric stretch in plane (ν3), which can be indexed as characteristic peaks for amorphous calcium carbonate (ACC).7,21,41,42 This result is consistent with our previous work14 that nanoparticles are obtained by an ACC-to-calcite transformation. It also shows that surfactant micelles have the ability to stabilize ACC as ACC can be found even after 48 h mineralization.
The n-hexane–aqueous interfaces we constructed should also be mentioned. Amphipathic surfactants in aqueous solution tend to aggregate at interfaces to minimize interfacial free energy. The concentration of surfactants at interfaces is higher than that of the bulk solution. Therefore, surfactant micelles on the substrates will be kept intact when the surfactant micelle-modified substrates are placed at the interfaces. Moreover, the use of the organic–inorganic interfaces is a way to mimic the biosystems because many organisms, including mollusks, echinoderms, calcisponges, and corals, form their hierarchical mineral skeleton out of calcium carbonate minerals in the organic–inorganic interfaces. In addition, organic–inorganic interfaces have been proved to have outstanding advantages over bulk aqueous solution in controlling the assembly of inorganic nanoparticles in previous works.43,44 Thus, this biomimetic approach will be promising in synthesizing other functional nanoparticles or nanodevices.
Conclusions
Combining the regulatory effect and the template role of surfactants, we use surfactant micelle-modified PET substrate to modulate the morphologies of CaCO3 at n-hexane–Ca(OH)2 saturated solution interfaces. After 48 h of crystallization, spherical and hexagonal CaCO3 crystals with hierarchical structure were obtained separately by different kinds of surfactants. During crystal growth, the assembled surfactant micelles not only contributed to stabilizing the assembly of nanoparticles, but also exerted vigorous control over the morphology of the products. From time-dependent experiments, we suggest that CaCO3 synthesis follows nanoparticle-mediated self-organization processes. Organic–inorganic liquid interfaces brought about controlled nucleation of calcite from ACC. Surfactant in solution induced formation of CaCO3 with well-defined orientation and morphology by specific adsorption. And, surfactant micelles on substrate act as a template which allows further growth and decides the final hierarchical structure of crystals. These findings will help in the better understanding of the formation mechanism of the unique CaCO3 morphologies. The biomimetic system will also contribute valuable information to biomineralization researches.Acknowledgements
This work is supported by the National Basic Research Program of China (973 Program) with no. 2011CB706900, the National Nature Science Foundation of China (nos. 50872149 and 50502003), the Scientific Research Foundation for Returned Scholars within the Ministry of Education of China, and the President Foundation of the Graduate University of the Chinese Academy of Sciences.Notes and references
- R. A. Metzler, I. W. Kim, K. Delak, J. S. Evans, D. Zhou, E. Beniash, F. Wilt, M. Abrecht, J.-W. Chiou, J. Guo, S. N. Coppersmith and P. U. P. A. Gilbert, Langmuir, 2008, 24, 2680–2687 CrossRef CAS PubMed.
- H. Colfen, Nat. Mater., 2010, 9, 960–961 CrossRef PubMed.
- W. Dong, C. Tu, W. Tao, Y. Zhou, G. Tong, Y. Zheng, Y. Li and D. Yan, Cryst. Growth Des., 2012, 12, 4053–4059 CAS.
- J. W. Nielsen, K. K. Sand, C. S. Pedersen, L. Z. Lakshtanov, J. R. Winther, M. Willemoës and S. L. S. Stipp, Cryst. Growth Des., 2012, 12, 4906–4910 CAS.
- G. Begum and R. K. Rana, Chem. Commun., 2012, 48, 8216–8218 RSC.
- Y. Fukui and K. Fujimoto, J. Mater. Chem., 2012, 22, 3493–3499 RSC.
- J. Ihli, Y.-Y. Kim, E. H. Noel and F. C. Meldrum, Adv. Funct. Mater., 2013, 23, 1575–1585 CrossRef CAS.
- L. Liu, D. He, G.-S. Wang and S.-H. Yu, Langmuir, 2011, 27, 7199–7206 CrossRef CAS PubMed.
- D. C. Bassett, B. Marelli, S. N. Nazhat and J. E. Barralet, Adv. Funct. Mater., 2012, 22, 3460–3469 CrossRef CAS.
- E. Asenath-Smith, H. Li, E. C. Keene, Z. W. Seh and L. A. Estroff, Adv. Funct. Mater., 2012, 22, 2891–2914 CrossRef CAS.
- H. Li and L. A. Estroff, J. Am. Chem. Soc., 2007, 129, 5480–5483 CrossRef CAS PubMed.
- J. Aizenberg, A. J. Black and G. M. Whitesides, J. Am. Chem. Soc., 1999, 121, 4500–4509 CrossRef CAS.
- B. Pokroy and J. Aizenberg, CrystEngComm, 2007, 9, 1219–1225 RSC.
- X. Liang, J. Xiang, F. Zhang, L. Xing, B. Song and S. Chen, Langmuir, 2009, 26, 5882–5888 CrossRef PubMed.
- A. S. Schenk, I. Zlotnikov, B. Pokroy, N. Gierlinger, A. Masic, P. Zaslansky, A. N. Fitch, O. Paris, T. H. Metzger, H. Cölfen, P. Fratzl and B. Aichmayer, Adv. Funct. Mater., 2012, 22, 4668–4676 CrossRef CAS.
- X. Wei, Y. Su, T. Wen, Z. Li, J. Yang and D. Wang, CrystEngComm, 2013, 15, 3417–3422 RSC.
- Z. Zhao, L. Zhang, H. Dai, Y. Du, X. Meng, R. Zhang, Y. Liu and J. Deng, Microporous Mesoporous Mater., 2011, 138, 191–199 CrossRef CAS PubMed.
- J. Aizenberg, N. Ilan, S. Weiner and L. Addadi, Connect. Tissue Res., 1996, 35, 17–23 Search PubMed.
- S. Elhadj, E. A. Salter, A. Wierzbicki, J. J. De Yoreo, N. Han and P. M. Dove, Cryst. Growth Des., 2005, 6, 197–201 Search PubMed.
- K. Naka, S.-C. Huang and Y. Chujo, Langmuir, 2006, 22, 7760–7767 CrossRef CAS PubMed.
- Y. Liu, Y. Cui, H. Mao and R. Guo, Cryst. Growth Des., 2012, 12, 4720–4726 CAS.
- Y.-Y. Kim, K. Ganesan, P. Yang, A. N. Kulak, S. Borukhin, S. Pechook, L. Ribeiro, R. Kröger, S. J. Eichhorn, S. P. Armes, B. Pokroy and F. C. Meldrum, Nat. Mater., 2011, 10, 890–896 CrossRef CAS PubMed.
- B. Xie, H. Shi, G. Liu, Y. Zhou, Y. Wang, Y. Zhao and D. Wang, Chem. Mater., 2008, 20, 3099–3104 CrossRef CAS.
- Y. Hou, M. Cao, M. Deng and Y. Wang, Langmuir, 2008, 24, 10572–10574 CrossRef CAS PubMed.
- K. S. Varanasi and S. Garoff, Langmuir, 2005, 21, 9932–9937 CrossRef CAS PubMed.
- H. Bock and K. E. Gubbins, Phys. Rev. Lett., 2004, 92, 135701 CrossRef CAS.
- R. Atkin and G. G. Warr, J. Am. Chem. Soc., 2005, 127, 11940–11941 CrossRef CAS PubMed.
- W. Mamdouh, H. Uji-i, J. S. Ladislaw, A. E. Dulcey, V. Percec, F. C. De Schryver and S. De Feyter, J. Am. Chem. Soc., 2005, 128, 317–325 CrossRef PubMed.
- A. Guerrero-Martínez, J. Pérez-Juste, E. Carbó-Argibay, G. Tardajos and L. M. Liz-Marzán, Angew. Chem., Int. Ed., 2009, 48, 9484–9488 CrossRef PubMed.
- K. S. Choi, E. W. McFarland and G. D. Stucky, Adv. Mater., 2003, 15, 2018–2021 CrossRef CAS.
- K.-S. Choi, H. C. Lichtenegger, G. D. Stucky and E. W. McFarland, J. Am. Chem. Soc., 2002, 124, 12402–12403 CrossRef CAS PubMed.
- P. Zhu, M. Teranishi, J. Xiang, Y. Masuda, W.-S. Seo and K. Koumoto, Thin Solid Films, 2005, 473, 351–356 CrossRef CAS PubMed.
- J. Xiang, P. Zhu, Y. Masuda and K. Koumoto, Langmuir, 2004, 20, 3278–3283 CrossRef CAS.
- M.-H. Park, Y. Ofir, B. Samanta and V. M. Rotello, Adv. Mater., 2009, 21, 2323–2327 CrossRef CAS.
- S. Manne and H. E. Gaub, Science, 1995, 270, 1480–1482 CAS.
- Y. I. Rabinovich, S. Pandey, D. O. Shah and B. M. Moudgil, Langmuir, 2006, 22, 6858–6862 CrossRef CAS PubMed.
- K. Shah, P. Chiu and S. B. Sinnott, J. Colloid Interface Sci., 2006, 296, 342–349 CrossRef CAS PubMed.
- J. Ihli, A. N. Kulak and F. C. Meldrum, Chem. Commun., 2013, 49, 3134–3136 RSC.
- P. Bots, L. G. Benning, J.-D. Rodriguez-Blanco, T. Roncal-Herrero and S. Shaw, Cryst. Growth Des., 2012, 12, 3806–3814 CAS.
- N. Gehrke, H. Cölfen, N. Pinna, M. Antonietti and N. Nassif, Cryst. Growth Des., 2005, 5, 1317–1319 CAS.
- L. Addadi, S. Raz and S. Weiner, Adv. Mater., 2003, 15, 959–970 CrossRef CAS.
- A. Sarkar, K. Dutta and S. Mahapatra, Cryst. Growth Des., 2012, 13, 204–211 Search PubMed.
- Y. Lin, H. Skaff, T. Emrick, A. D. Dinsmore and T. P. Russell, Science, 2003, 299, 226–229 CrossRef CAS PubMed.
- M. X. Luo, Y. M. Song and L. L. Dai, J. Chem. Phys., 2009, 131, 194703 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The XRD pattern of blank PET substrate. The SEM image of crystals grown at the interface without surfactant in the inorganic phase for 48 h. See DOI: 10.1039/c4ra02694b |
| This journal is © The Royal Society of Chemistry 2014 |





