Fe2MnSixGe1−x: influence thermoelectric properties of varying the germanium content
A. H. Reshak*ab
aNew Technologies – Research Centre, University of West Bohemia, Univerzitni 8, 306 14 Pilsen, Czech Republic. E-mail: maalidph@yahoo.co.uk; Tel: +420 777 729 583
bCenter of Excellence Geopolymer and Green Technology, School of Material Engineering, Malaysia
First published on 11th August 2014
Abstract
The semi-classical Boltzmann theory, as implemented in the BoltzTraP code, was used to study the influence of varying the germanium content on the thermoelectric properties of the Heusler compounds, Fe2MnSi and Fe2MnGe. The electrical conductivity (σ/τ), the Seebeck coefficient (S), the electronic power factor (S2σ), the electronic thermal conductivity (κe), the electronic heat capacity cel(Tel), and the Hall coefficient (RH), as a function of temperature at certain values of chemical potential (μ) with constant relaxation time (τ), were evaluated on the basis of the calculated band structure using the standard Boltzmann kinetic transport theory and the rigid band approach. The increase/reduction in the electrical conductivity (σ = neμ) of Fe2MnSixGe1−x alloys is attributed to the density of charge carriers (n) and their mobility (μ = eτ/me). The S for Fe2MnGe is negative over the entire temperature range, which represents the n-type concentration. Whereas Fe2MnSi shows a positive S up to 250 K and then drops to negative values, which confirms the existence of the p-type concentration between 100–250 K. Fe2MnSi0.25Ge0.75/Fe2MnSi0.5Ge0.5/Fe2MnSi0.75Ge0.25 possess positive S up to 270/230/320 K and then drop to negative values. The power factor of Fe2MnGe rapidly increases with increasing temperature, while for Fe2MnSi it is zero up to 300 K, and then rapidly increases with increasing temperature. The S2σ of Fe2MnSi0.25Ge0.75 is zero between 250–350 K, whereas Fe2MnSi0.5Ge0.5 possesses a zero S2σ of up to 320 K. Fe2MnSi0.75Ge0.25 has a zero S2σ between 200 and 500 K. The electronic thermal conductivity (κe) and the electronic heat capacity cel(Tel) increases with increasing temperature. The parent compounds (Fe2MnGe and Fe2MnSi) show the highest positive value of the Hall coefficient RH at 100 K, and then drop to negative values at 260 K. On the other hand, the RH for Fe2MnSi0.25Ge0.75, Fe2MnSi0.5Ge0.5 and Fe2MnSi0.75Ge0.25 alloys exhibit negative RH along the temperature scale. The behavior of RH is attributed to the concentration of the charge carriers and their mobility.
1. Introduction
Half metallic compounds have received a significant amount of attention1–13 because of the spin degree of freedom in electronics.14–17 Significant effort has been paid to understanding the mechanism behind half-metallic magnetism and to study its implications on various physical properties.18,19 The field of spin-based electronics (spintronics) aims to exploit a large class of emerging materials, such as ferromagnetic semiconductors,20,21 high temperature superconductors,22 organic ferromagnets,23,24 organic semiconductors,25 and carbon nanotubes26,27 based devices to bring novel functionalities to traditional devices. In other areas within spintronics, as high as possible a degree of spin polarisation is required, for which half metals are considered the best options. Half-metallic compounds behave as metals in one spin direction, and behave as semiconductors or insulators in the opposite spin direction.In 1983, De Groot et al.28,29 discovered the half-metallic ferromagnetic (HMF). Ever since this discovery, extensive studies have been carried out and many HMF compounds have been theoretically predicted, and some of them experimentally confirmed.30–32 It is well known that the most important candidates for 100% spin polarization are semi-Heusler alloys,19,28,29,33–35 zinc-blende structured materials,36,37 semi-metallic magnetic oxides CrO2 and Fe3O4,11,38,39 and full Heusler alloys.9,40 Fe2MnSi1−xGex alloys are considered as very interesting HMF materials.41–43 Fe2MnSi1−xGex alloys can be successfully applied to highly efficient spin injection and detection through Schottky tunnel barriers in group-IV semiconductor devices.
Epitaxial Fe2MnSi thin films have a Curie temperature of about 210 K, which is much lower than room temperature;44 however, only limited experimental work has been done on these materials. Zhang et al. have studied Fe2MnSi1−xGex alloys and found there is no significant change in the Curie temperature and the spontaneous magnetization, with change the Ge content in the single-phase L21 compounds. There is no field-induced transition and the magneto-caloric effect is rather small throughout the Fe2MnSi1−xGex series.45 In addition, in the L21-type structure, both Fe2MnGe and Fe2MnSi have a Curie temperature of around 250 K.45 Therefore, it is worth studying the transport properties of Fe2MnSixGe1−x alloys by varying the germanium content between 0.0 and 1.0, in steps of 0.25 atoms.
The rest of the paper is organized as; the structural aspects and the computational details are presented in Section 2. The results and discussion are demonstrated in Section 3. Section 4 summarizes the results.
2. Structural aspects and computational details
To study the influence of varying the germanium content on the thermoelectric properties of the Heusler compounds Fe2MnSi and Fe2MnGe, the semi-classical Boltzmann theory as implemented in the BoltzTraP code46 was used. The thermoelectric transport tensors can be evaluated on the basis of the calculated band structure using the standard Boltzmann kinetic transport theory and the rigid band approach.47 The electrical conductivity σαβ, the Seebeck coefficient Sαβ, and the electronic thermal conductivity k0αβ tensors are the main transport properties.In this work, the germanium content in Fe2MnSixGe1−x alloys was varied between 0.0 and 1.0, in steps of 0.25 atoms. The parent compounds crystallize in the L21 structure, which consists of four face-centered cubic sublattices with space Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The L21 phase is a cubic superstructure of four interpenetrating fcc sublattices, A, B, C, and D, centered at (0 0 0), (1/4 1/4 1/4), (1/2 1/2 1/2), and (3/4 3/4 3/4). Each A atom is at the center of a cube of four B atoms and four D atoms (Fig. 1). To trace the variation in the composition, we employed the supercell approach. The electronic structure was calculated using the full potential linear augmented plane wave, plus the local orbitals method, as implemented in WIEN2k code48 with the Engel–Vosko generalized gradient approximation for the exchange–correlation potential. The structures were fully relaxed by minimizing the forces acting on each atom. A mesh of 5000 k-points in the irreducible Brillouin zone (IBZ) for binary, as well as for ternary alloys, was used for calculating the thermoelectric properties. The Kmax was set at 9.0/RMT and made the expansion up to l = 10 in the muffin tins spheres. The convergence of the total energy in the self-consistent calculations was taken with respect to the total charge of the system with a tolerance 0.0001 electron charges.
m. The L21 phase is a cubic superstructure of four interpenetrating fcc sublattices, A, B, C, and D, centered at (0 0 0), (1/4 1/4 1/4), (1/2 1/2 1/2), and (3/4 3/4 3/4). Each A atom is at the center of a cube of four B atoms and four D atoms (Fig. 1). To trace the variation in the composition, we employed the supercell approach. The electronic structure was calculated using the full potential linear augmented plane wave, plus the local orbitals method, as implemented in WIEN2k code48 with the Engel–Vosko generalized gradient approximation for the exchange–correlation potential. The structures were fully relaxed by minimizing the forces acting on each atom. A mesh of 5000 k-points in the irreducible Brillouin zone (IBZ) for binary, as well as for ternary alloys, was used for calculating the thermoelectric properties. The Kmax was set at 9.0/RMT and made the expansion up to l = 10 in the muffin tins spheres. The convergence of the total energy in the self-consistent calculations was taken with respect to the total charge of the system with a tolerance 0.0001 electron charges.
3. Results and discussion
3.1. Salient features of the electronic band structures
The calculated electronic band structure of Fe2MnSixGe1−x (x = 0.0, 0.25, 0.5, 0.75, 1.0) alloys along the high symmetry point in the first BZ are represented in Fig. 2a–e. These figures suggest that the investigated alloys are metallic, as it is clear that there exists some bands that controlled the overlapping around the Fermi energy (EF). The density of states at EF is determined by the overlap between the valence and conduction bands. This overlap is strong enough, indicating a metallic origin with different values of DOS at EF, N(EF) (Table 1). The electronic specific heat coefficient (γ), which is function of the density of states, can be calculated using the expression,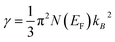 , where N(EF) is the density of states at EF, and kB is the Boltzmann constant. The calculated density of states at the Fermi energy N(EF) enables us to calculate the bare electronic specific heat coefficient (Table 1). It is clear that substituting 0.25 Ge atoms by Si atoms leads to increasing the metallic nature of Fe2MnSi0.25Ge0.75 by 3.7% with respect to Fe2MnGe, while substituting 0.5 Ge atoms by Si atoms causes an increase in the metallic nature of Fe2MnSi0.5Ge0.5 by 1.9% with respect to Fe2MnGe. Further increasing the continent of Si atoms leads to increasing the metallic nature of Fe2MnSi0.75Ge0.25 by 3% with respect to Fe2MnGe, and finally substituting Ge by Si causes a reduction of the metallic nature of Fe2MnSi by 0.9% with respect to Fe2MnGe. The same trends were observed for the electronic specific heat coefficient (γ).
, where N(EF) is the density of states at EF, and kB is the Boltzmann constant. The calculated density of states at the Fermi energy N(EF) enables us to calculate the bare electronic specific heat coefficient (Table 1). It is clear that substituting 0.25 Ge atoms by Si atoms leads to increasing the metallic nature of Fe2MnSi0.25Ge0.75 by 3.7% with respect to Fe2MnGe, while substituting 0.5 Ge atoms by Si atoms causes an increase in the metallic nature of Fe2MnSi0.5Ge0.5 by 1.9% with respect to Fe2MnGe. Further increasing the continent of Si atoms leads to increasing the metallic nature of Fe2MnSi0.75Ge0.25 by 3% with respect to Fe2MnGe, and finally substituting Ge by Si causes a reduction of the metallic nature of Fe2MnSi by 0.9% with respect to Fe2MnGe. The same trends were observed for the electronic specific heat coefficient (γ).
 | ||
| Fig. 2 (a–e) Calculated electronic band structure of Fe2MnSixGe1−x (x = 0.0, 0.25, 0.5, 0.75 and 1.0) alloys. | ||
| Fe2MnGe | Fe2MnSi0.25Ge0.75 | Fe2MnSi0.5Ge0.5 | Fe2MnSi0.75Ge0.25 | Fe2MnSi | |
|---|---|---|---|---|---|
| N(EF)states per eV cell | 17.11 | 65.0 | 33.93 | 58.15 | 16.07 |
| γ (mJ mol−1 K−2) | 2.96 | 11.27 | 5.88 | 10.08 | 2.78 |
3.2. Transport properties
Fig. 3a illustrates the influence of substituting Ge by Si (in steps of 0.25 atoms) on the electrical conductivity (σ/τ) of the Heusler compounds Fe2MnGe and Fe2MnSi. In all cases, we notice that σ/τ increases with temperature, and it is clear that the increasing rate is dependent on the concentration of Ge and Si atoms. The end compounds (Fe2MnGe and Fe2MnSi) show the highest value for Fe2MnSi of about 1.49 × 1020 (Ω ms)−1 at 100 K and 1.9 × 1020 (Ω ms)−1 at 800 K; while for Fe2MnGe, it is 1.4 × 1020 (Ω ms)−1 at 100 K and 1.82 × 1020 (Ω ms)−1 at 800 K. With substituting 0.25 Ge by Si (Fe2MnSi0.25Ge0.75), we notice that the value of σ/τ drops to the lowest values along the whole temperature range. Then, increasing the content of Si atoms to be equal to Ge atoms (Fe2MnSi0.5Ge0.5) causes an increase in the electrical conductivity to its maximum value of about 1.1 × 1020 (Ω ms)−1 at 100 K and 1.4 × 1020 (Ω ms)−1 at 800 K. Further increasing the content of Si atoms (Fe2MnSi0.75Ge0.25) leads to a reduction in the electrical conductivity to 0.9 × 1020 (Ω ms)−1 at 100 K and 1.3 × 1020 (Ω ms)−1 at 800 K. From above, we can conclude that the increase/reduction in the electrical conductivity of Fe2MnSixGe1−x alloys is attributed to the density of charge carriers (n) and their mobility (μ = eτ/me), since the electrical conductivity (σ = neμ) is related to the density of charge carriers and their mobility.The Seebeck coefficient Sαβ and the electrical conductivity σαβ tensors can be written as:47,49
From the above formulas, it is clear that the Seebeck coefficient is inversely proportional to the electrical conductivity, and that these quantities are functions of temperature (T) and chemical potential (μ).47,49
Fig. 3b illustrates the Seebeck coefficient (S) as a function of temperature at certain values of chemical potential (μ). Following Fig. 3b, one can see that the S for Fe2MnGe is negative over the entire temperature range, which represents the n-type concentration. It is clear that S reduces rapidly with increasing the temperature, and it possesses a maximum value at 100 K (−0.2 × 10−5 V K−1) and the lowest value at 800 K. Whereas, Fe2MnSi shows positive S up to 250 K, and then drop to negative values, which confirms the existence of the p-type concentration between 100 K and 250 K. When we substitute 0.25 Ge atoms by Si (Fe2MnSi0.25Ge0.75), we notice that the Seebeck coefficient shows the maximum positive value at 100 K (0.9 × 10−5 V K−1), and then reduces with increasing the temperature up to 270 K. Above this temperature, S drops to negative values, to reach the lower value of about (−0.7 × 10−5 V K−1) at 800 K.
With substituting half the content of Ge atoms by the same content of Si atoms (Fe2MnSi0.5Ge0.5), we notice that the Seebeck coefficient reduced, to show a maximum value at 100 K of about (0.2 × 10−5 V K−1), and then drops to negative values at 230 K to reach −0.8 × 10−5 V K−1 at 800 K. In further increasing the Si content at the cost of the Ge content (Fe2MnSi0.75Ge0.25), the value of the Seebeck coefficient at 100 K increases to 0.6 × 10−5 V K−1 with respect to the value of S obtained for Fe2MnSi0.5Ge0.5, and then drops to negative values at 320 K.
Fig. 3c shows the electronic power factor (S2σ) verses the temperature at certain values of chemical potential (μ) and constant relaxation time (τ). We notice that the power factor of Fe2MnGe rapidly increases with increasing the temperature, to reach the maximum value (9 × 1010 W m−1 K−2 s−1) at 800 K. While for Fe2MnSi, the power factor is zero up to 300 K, and then rapidly increases with increasing temperature to reach the maximum value (3.9 × 1010 W m−1 K−2 s−1) at 800 K. With replacing 0.25 Ge atoms by Si (Fe2MnSi0.25Ge0.75), the power factor increases with respect to all other concentrations, and shows its maximum value of about (0.8 × 1010 W m−1 K−2 s−1) at 100 K, and then reduces with increasing temperature to reach a zero value of S2σ between 250 K and 350 K. Above this temperature range, S2σ increases with increasing temperature, to reach 0.2 × 1010 W m−1 K−2 s−1 at 800 K. Substituting 0.5 of Ge atoms by Si (Fe2MnSi0.5Ge0.5) causes the power factor to drop to zero up to 320 K. Then above 350 K, the power factor increases with increasing temperature, to reach 1.0 × 1010 W m−1 K−2 s−1 at 800 K. In the last case (Fe2MnSi0.75Ge0.25), when we reduced the content of Ge to 0.25, the power factor became 0.4 × 1010 W m−1 K−2 s−1 at 100 K, and then dropped to zero between 200 K and 500 K, while above 500 K, the Seebeck coefficient showed an insignificant increase to reach 0.2 × 1010 W m−1 K−2 s−1 at 800 K.
The electronic thermal conductivity (κe) can be estimated from the electrical conductivity (σ) using Wiedemann–Franz's law. Fig. 3d illustrate κe for Fe2MnSixGe1−x alloys as a function of temperature at certain values of chemical potential. We notice that for all Fe2MnSixGe1−x alloys, κe increases rapidly with increasing temperature. The end compound Fe2MnSi shows the highest value of κe between 100 K and 800 K. While κe for Fe2MnGe lies directly below that of Fe2MnSi exhibiting the same trend. When we replace a quarter of the Ge atoms by Si (Fe2MnSi0.25Ge0.75), the thermal conductivity drops down to the lowest value along the temperature scale. With increasing the content of Si atoms to be equal to that of Ge atoms (Fe2MnSi0.5Ge0.5), we can see that the κe values increases along the temperature scale. Substituting more Ge atoms by Si atoms (Fe2MnSi0.75Ge0.25) leads to a reduction in the values of the electronic thermal conductivity with respect to that of Fe2MnSi0.5Ge0.5. Following Fig. 3d, we can conclude that the electronic thermal conductivity is very sensitive to the density of the charge carriers and their mobility. The thermal conductivity has contributions from the lattice and electrons, but BoltzTraP calculates only the electronic part. In the absence of any calculations or measurements of the lattice thermal conductivity, it is difficult to confirm which alloy will have the largest figure of merit (FOM).
The electronic heat capacities cel(Tel) for Fe2MnSixGe1−x alloys as a function of temperature at certain values of chemical potential with constant relaxation time are plotted in Fig. 3e. Here, we consider only the electronic contribution to the specific heat, because there is a linear relationship between the electronic specific heat and temperature i.e., cel(Tel) = γTel, where γ = the Sommerfeld coefficient.50,51 The electrons are excited to the upper empty space and there is a smearing of Fermi below the Fermi level, which together contribute to the heat capacity. From Fig. 3e, we can see that the heat capacity for the end compounds (Fe2MnGe and Fe2MnSi) slowly increases with temperature up to 500 K, and then reach saturation up to 800 K. Fe2MnSi0.25Ge0.75 alloy shows higher values of cel(Tel) compared to the parents. Increasing the Si content (Fe2MnSi0.5Ge0.5) causes a reduction in the cel(Tel) lower than that of Fe2MnSi0.25Ge0.75 but higher than that of the parents. Further increasing the Si content (Fe2MnSi0.75Ge0.25) causes an increase in cel(Tel), showing the highest value among the others. It is clear from Fig. 3e that the electronic heat capacity of Fe2MnSixGe1−x alloys obey the Debye approximation (T3), also called the “anharmonic approximation”.52
Fig. 3f presents the Hall coefficient RH as a function of temperature; its value depends on the type, number, and properties of the charge carriers that constitute the current. It is clear that the parents compounds (Fe2MnGe and Fe2MnSi) show the highest positive value of RH at 100 K, which then rapidly reduces with increasing temperature to cross the zero line and then reach negative values at 260 K. Above 360 K, the RH for the parents compounds is almost saturated. The RH for Fe2MnSi0.25Ge0.75, Fe2MnSi0.5Ge0.5 and Fe2MnSi0.75Ge0.25 alloys exhibits negative RH along the temperature scale, which is attributed to the concentration of the charge carriers and their mobility.
To the best of our knowledge, there are no previous experimental data or theoretical results for the thermoelectric properties of the investigated materials available in literature to make a meaningful comparison. We would like to mention here that in our previous works53–59 we calculated the band gap, bond lengths, bond angles, and linear and nonlinear optical susceptibilities using the FPLAPW method on several systems whose energy band gap, bond lengths, bond angles, and linear and nonlinear optical susceptibilities are known experimentally. We achieved very good agreement with the experimental data. Thus, we believe that our calculations reported in this paper could produce very accurate and reliable results, which therefore confirms the accuracy of the method used.
4. Conclusions
The full potential linear augmented plane wave plus local orbitals method, as implemented in WIEN2k code with the Engel–Vosko generalized gradient approximation for the exchange–correlation potential, were used to calculated band structure for Fe2MnSixGe1−x (x = 0.0, 0.25, 0.5, 0.75 and 1.0) alloys. Based on the calculated band structure, the semi-classical Boltzmann theory as implemented in the BoltzTraP code was used to study the influence of varying the germanium content on the thermoelectric properties of Fe2MnSixGe1−x alloys as a function of temperature at certain values of chemical potential (μ) with a constant relaxation time (τ). The increase/reduction in the electrical conductivity (σ = neμ) of Fe2MnSixGe1−x alloys is attributed to the density of charge carriers (n) and their mobility (μ = eτ/me). The parent Fe2MnGe compound exhibits negative S over the entire temperature range, which confirms the existence of an n-type carrier. While Fe2MnSi shows positive S up to 250 K, and then drops to negative values, to represent the p-type charge carrier between 100 K and 250 K. The alloys Fe2MnSi0.25Ge0.75/Fe2MnSi0.5Ge0.5/Fe2MnSi0.75Ge0.25 all possess positive S up to 270/230/320 K. Above these temperatures, the Seebeck coefficient exhibits negative values. The power factor of Fe2MnGe rapidly increases with increasing temperature, while for Fe2MnSi it is zero up to 300 K, and then rapidly increases with increasing temperature. Fe2MnSi0.25Ge0.75 has a zero power factor between 250 K and 350 K. While Fe2MnSi0.5Ge0.5 shows a zero power factor up to 320 K. The Fe2MnSi0.75Ge0.25 alloy shows a zero power factor between 200 K and 500 K. The electronic thermal conductivity (κe) and the electronic heat capacity cel(Tel) increase with increasing temperature. The parent compounds show the highest positive value of RH at 100 K, and then drop to negative values at 260 K. The alloys Fe2MnSi0.25Ge0.75, Fe2MnSi0.5Ge0.5 and Fe2MnSi0.75Ge0.25 alloys exhibit negative RH along the temperature scale. The positive and negative behavior of RH is attributed to the concentration of the charge carriers and their mobility.Acknowledgements
The result was developed within the CENTEM project, reg. no. CZ.1.05/2.1.00/03.0088, co-funded by the ERDF as part of the Ministry of Education, Youth and Sports OP RDI program. Computational resources were provided by MetaCentrum (LM2010005) and CERIT-SC (CZ.1.05/3.2.00/08.0144) infrastructures.References
- K. E. H. M. Hanssen, P. E. Mijnarends, L. P. L. M. Rabou and K. H. J. Buschow, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 42, 1533 CrossRef CAS.
- H. Kino, F. Aryasetiawanx, I. Solovyer, T. Miyake, T. Ohno and K. Terakura, Phys. B, 2003, 329, 858 CrossRef.
- R. A. de Groot, Phys. B, 1991, 172, 45 CrossRef CAS.
- J. H. Wijngaard, C. haas and R. A. de Groot, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 9318 CrossRef CAS.
- S. Chadov, J. Minar, H. Ebert, A. Perlov, L. Chioncel, M. I. Katsnelson and A. I. Lichtenstein, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 140411 CrossRef.
- B. I. Min, T. Oguchi, H. J. F. Jansen and A. J. Freeman, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 324 CrossRef CAS.
- G. A. de Wijs and R. A. de Groot, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 020402 CrossRef.
- E. Kisker, C. Carbone, C. F. Flipse and E. F. Wassermann, J. Magn. Magn. Mater., 1987, 70, 21 CrossRef CAS.
- I. Galanakis, P. H. Dederichs and N. Papanikolaou, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 174429 CrossRef.
- K. Nagao, M. Shirai and Y. Miura, J. Phys.: Condens. Matter, 2004, 16, 5725 CrossRef.
- K. Schwarz, J. Phys. F: Met. Phys., 1986, 16, 211 CrossRef.
- I. I. Mazin, D. J. Singh and C. Ambrosch-Draxl, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 411 CrossRef CAS.
- G. L. Zhao, J. Callaway and M. Hayashibara, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 15781 CrossRef CAS.
- G. A. Prinz, Science, 1998, 282, 1660 CrossRef CAS.
- S. A. Wolf, D. D. Awschalom, R. A. Buhrman, J. M. Daughton, S. von Molnar, M. L. Roukes, A. Y. Chtchelkanova and D. M. Treger, Science, 2001, 294, 1488 CrossRef CAS PubMed.
- I. Zutic, J. Fabian and S. Das Sarma, Rev. Mod. Phys., 2004, 76, 323 CrossRef CAS.
- D. D. Awschalom and M. E. Flatte, Nat. Phys., 2007, 3, 153 CrossRef CAS.
- T. Akimoto, Y. Moritomo, A. Nakamura and N. Furukawa, Phys. Rev. Lett., 2000, 85, 3914 CrossRef CAS.
- C. M. Fang, G. A. de Wijs and R. A. de Groot, J. Appl. Phys., 2002, 91, 8340 CrossRef CAS PubMed.
- H. Ohno, Science, 1998, 281, 951 CrossRef CAS.
- S. J. Pearton, C. R. Abernathy, M. E. Overberg, G. T. Thaler, D. P. Norton, N. Theodoropoulou, A. F. Hebard, Y. D. Park, F. Ren, J. Kim and L. A. Boatner, J. Appl. Phys., 2003, 93, 1 CrossRef CAS PubMed.
- A. M. Goldman, V. Vasko, P. Kraus, K. Nikolaev and V. A. Larkin, J. Magn. Magn. Mater., 1999, 200, 69 CrossRef CAS.
- V. Dediu, M. Murgia, F. C. Matacotta, C. Taliani and S. Barbanera, Solid State Commun., 2002, 122, 181 CrossRef CAS.
- A. J. Epstein, MRS Bull., 2003, 28, 492 CrossRef CAS.
- D. A. Pejakovic, C. Kitamura, J. S. Miller and A. J. Epstein, Phys. Rev. Lett., 2002, 88, 057202 CrossRef.
- B. Zhao, I. Monch, H. Vinzelberg, T. Muhl and C. M. Schneider, Appl. Phys. Lett., 2002, 80, 3144 CrossRef CAS PubMed.
- K. Tsukagoshi, B. W. Alphenaar and H. Ago, Nature, 1999, 401, 572 CrossRef CAS PubMed.
- R. A. de Groot, F. M. Mueller, P. G. van Engen and K. H. J. Buschow, Phys. Rev. Lett., 1983, 50, 2024 CrossRef CAS.
- R. A. de Groot, F. M. Mueller, P. G. van Engen and K. H. J. Buschow, J. Appl. Phys., 1984, 55, 2151 CrossRef CAS PubMed.
- J. W. Dong, L. C. Chen, J. C. Palmstom, R. D. James and S. Mckernan, Appl. Phys. Lett., 1999, 75, 1443 CrossRef CAS PubMed.
- S. M. Watta, S. Wirth, S. Von Molnar, A. Barry and J. M. D. Coey, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 9621 CrossRef.
- F. J. Jedema, A. T. Filip and B. van Wees, Nature, 2001, 410, 345 CrossRef CAS PubMed.
- J. Kubler, Phys. B, 1984, 127, 257 Search PubMed.
- K. E. H. M. Hanssen and P. E. Mijnarends, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 34, 5009 CrossRef CAS.
- I. Galanakis, S. Ostanin, M. Alouani, H. Dreysse and J. M. Wills, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 4093 CrossRef CAS.
- B. G. Liu, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 172411 CrossRef.
- I. Galanakis, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 012406 CrossRef.
- S. P. Lewis, P. B. Allen and T. Sasaki, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 10253 CrossRef CAS.
- R. A. de Groot and K. H. J. Buschow, J. Magn. Magn. Mater., 1986, 54, 1377 CrossRef.
- I. Galanakis, J. Phys.: Condens. Matter, 2002, 14, 6329 CrossRef CAS.
- S. Fujii, S. Ishida and S. Asano, J. Phys. Soc. Jpn., 1995, 64, 185 CrossRef CAS.
- L. Hongzhi, Z. Zhiyong, M. Li, X. Shifeng, L. Heyan, Q. Jingping, L. Yangxian and W. Guangheng, J. Phys. D: Appl. Phys., 2007, 40, 7121 CrossRef.
- B. Hamad, J. Khalifeh, I. Adu Aljarayesh, C. Demangeat, H.-B. Luo and Q.-M. Hu, J. Appl. Phys., 2010, 107, 09311 CrossRef PubMed.
- K. Ueda, K. Hamaya, K. Yamamoto, Y. Ando, T. Sadoh, Y. Maeda and M. Miyao, Appl. Phys. Lett., 2008, 93, 112108 CrossRef PubMed.
- L. Zhang, E. Bruck, O. Tegus, H. H. J. Buschow and F. R. de Boer, Phys. B, 2003, 328, 295 CrossRef CAS.
- G. K. H. Madsen and D. J. Singh, cond-mat.mtrl-sci., 8 February 2006.
- G. K. H. Madsen and D. J. Singh, Comput. Phys. Commun., 2006, 175, 67–71 CrossRef CAS PubMed.
- P. Blaha, K. Schwarz, G. Madsen, D. Kvasnika and K. Luitz, WIEN2k, Technical Universität Wien, Austria, 2001, ISBN 3-9501031-1-2 Search PubMed.
- P. Hua, W. C. Lei, L. J. Chao, Z. Rui-Zhi, W. Hong-Chao and S. Y. Chin, Phys. B, 2011, 20(4), 046103 Search PubMed.
- N. W. Ashcroft and N. D. Mermin, Solid State Physics, Holt, Rinehart and Winston, New York, 1976 Search PubMed.
- Z. Zhang, Nano/Microscale Heat Transfer, McGraw-Hill, New York, 2007 Search PubMed.
- C. Dong, et al., Chin. Phys. B DOI:1674-1056/2009/18(02)/0738-06.
- A. H. Reshak, S. Auluck and I. V. Kityk, J. Phys.: Condens. Matter, 2008, 20, 145209 CrossRef.
- A. H. Reshak, S. Auluck and I. V. Kityk, J. Solid State Chem., 2008, 181, 789–795 CrossRef CAS PubMed.
- A. H. Reshak, J. Chem. Phys., 2006, 124, 104707 CrossRef PubMed.
- A. H. Reshak, Eur. Phys. J. B, 2005, 47, 503–508 CrossRef CAS.
- A. H. Reshak, I. V. Kityk and S. Auluck, J. Phys. Chem. B, 2010, 114, 16705–16712 CrossRef CAS PubMed.
- A. H. Reshak, S. Auluck, D. Stys, I. V. Kityk, H. Kamarudin, J. Berdowski and Z. Tylczynskif, J. Mater. Chem., 2011, 21, 17219 RSC.
- A. H. Reshak, M. Piasecki, S. Auluck, I. V. Kityk, R. Khenata, B. Andriyevsky, C. Cobet, N. Esser, A. Majchrowski, M. S'wirkowicz, R. Diduszko and W. Szyrski, J. Phys. Chem. B, 2009, 113(46), 15237 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |




