Synthesis and self-assembly behavior of organic–inorganic macrocyclic molecular brushes composed of macrocyclic oligomeric silsesquioxane and poly(N-isopropylacrylamide)
Abstract
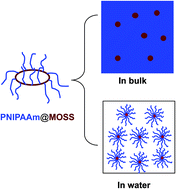
The novel organic–inorganic molecular brush composed of macrocyclic oligomeric silsesquioxane (MOSS) and poly(N-isopropylacrylamide) (PNIPAAm) (denoted by PNIPAAm@MOSS) was synthesized via the atom transfer radical polymerization (ATRP) approach. In bulk, the organic–inorganic molecular brushes were microphase-separated; the spherical MOSS microdomains with the diameter of 10–50 nm were dispersed into a continuous PNIPAAm matrix. Depending on the lengths of PNIPAAm chains, the PNIPAAm@MOSS molecular brushes were capable of self-assembling into cylindrical or spherical nano-objects in aqueous solutions as evidenced by transmission election microscopy (TEM) and dynamic light scattering (DLS). Both micro-differential scanning calorimetry (Micro-DSC) and ultraviolet-visible spectroscopy showed that the MOSS backbones exerted significant restriction of coil-to-globule transition of PNIPAAm chains.

 Please wait while we load your content...
Please wait while we load your content...