Hollow zinc oxide microspheres functionalized by Au nanoparticles for gas sensors
Abstract
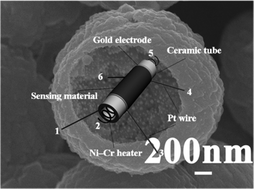
We present a facile one-step template-free route for the synthesis of homogeneous Au-loaded ZnO hollow spheres composed of nanoparticles. The synthesis was performed at a relatively low temperature (90 °C) with short reaction duration (40 min). Field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM) and energy dispersive X-ray spectrometry (EDX) analysis revealed that Au nanoparticles were homogeneously embedded into ZnO spherical shell, which was composed of nano-sized primary particles. Furthermore, to investigate the influence of Au nanoparticles on the sensor performance, the gas sensing properties of Au-loaded ZnO were studied. A comparative gas sensing investigation between the Au-loaded ZnO and pure ZnO hollow spheres was performed to display the superior sensing properties of the loaded samples. As expected, the sensor using 1.0 mol% Au-loaded ZnO exhibited a high response and fast response and recovery properties to ethanol, which could be attributed to the catalytic effect of Au.

 Please wait while we load your content...
Please wait while we load your content...