DOI:
10.1039/C4RA02519A
(Paper)
RSC Adv., 2014,
4, 26389-26397
Construction of C(sp2)–S and C(sp2)–Se bonds via a silver(I)-mediated coupling reaction of heterocyclic ketene aminals with diaryl dichalcogenides†
Received
22nd March 2014
, Accepted 27th May 2014
First published on 27th May 2014
Abstract
A novel silver(I)-mediated direct coupling reaction using heterocyclic ketene aminals (HKAs) and diaryl dichalcogenides for the construction of C(sp2)–S and C(sp2)–Se bonds was reported. The transformation involves a variety of functionalized substrates, leading to α-arylthio and α-phenylselanyl HKAs in a mild, facile and efficient way with high regioselectivity and excellent yields. The broad scope of the starting materials enhanced the chemo-diversity of the target materials, thus affording a number of potential applications in the synthesis of heterocycles and its relevant medicinal chemistry.
Introduction
A transition-metal-catalyzed coupling reaction for the formation of carbon–heteroatom bonds is one of the most active contemporary topics in modern synthetic chemistry.1 The development of concise and sustainable procedures, as well as the discovery of novel and efficient transition-metal catalysts, have been a driving force in the construction of selective synthetic methods. Among the plethoric formalism of carbon–heteroatom bonds, in particular, the sp2 hybrid carbon–chalcogen bonds2 have enjoyed prominent standing in fields such as total synthesis,3 medicinal chemistry,4 organometallic chemistry,5 chemical biology,6 and supramolecular chemistry.7 Despite remarkable progress in direct transition-metal-catalyzed coupling reactions of aromatic systems with chalcogen,8 olefin systems with chalcogen have been hardly explored.9
In the past few decades, stereoselective and regioselective synthesis focusing on heterocycles frequently found in natural products, pharmaceuticals, and dyes has played a significant role in many chemical branches, leading to the rapid development of widely used synthons. For example, there is a lot of research effort focused on concise and efficient access of HKAs to highly functionalized target materials.10–19 The structure of HKAs is shown as Fig. 1. Due to the conjugation of electro-donating amino groups and the electron-withdrawing carbonyl group, the double bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of HKAs is highly polarized.19 This leads to higher electron density of the α-carbon (C3) than that of the secondary amino groups (N1 and N5). Consequently, the substituted targets of the α-carbon have been obtained with high selectivity via alkylation,20 acylation,21 glycosation,22 halogenations,23 and nitration reactions.24 However, to the best of our knowledge, no information is available on the formation of C–chalcogen bonds via the coupling reaction of HKAs with chalcogen. Herein, we report the first example of an AgOAc-mediated direct coupling reaction of HKAs 1 with diaryl dichalcogenides 2 that leads to the regioselective functionalization of the α-carbon with excellent yields (83–98%) by a facile post-treatment protocol. The scope of the reaction encompasses HKAs and diaryl dichalcogenides bearing different sized rings, substitution patterns, and functional groups, which suggests that these novel synthons will be suitable for further synthesis of diverse heterocyclic architectures.
C) of HKAs is highly polarized.19 This leads to higher electron density of the α-carbon (C3) than that of the secondary amino groups (N1 and N5). Consequently, the substituted targets of the α-carbon have been obtained with high selectivity via alkylation,20 acylation,21 glycosation,22 halogenations,23 and nitration reactions.24 However, to the best of our knowledge, no information is available on the formation of C–chalcogen bonds via the coupling reaction of HKAs with chalcogen. Herein, we report the first example of an AgOAc-mediated direct coupling reaction of HKAs 1 with diaryl dichalcogenides 2 that leads to the regioselective functionalization of the α-carbon with excellent yields (83–98%) by a facile post-treatment protocol. The scope of the reaction encompasses HKAs and diaryl dichalcogenides bearing different sized rings, substitution patterns, and functional groups, which suggests that these novel synthons will be suitable for further synthesis of diverse heterocyclic architectures.
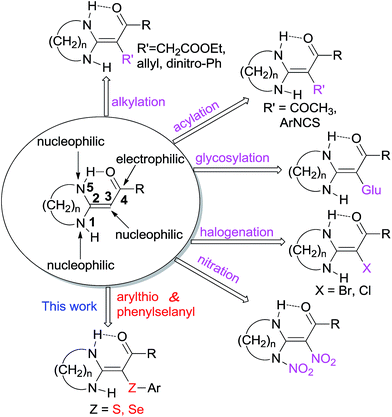 |
| | Fig. 1 Regioselective reactions of the α-carbon of HKAs. | |
Results and discussion
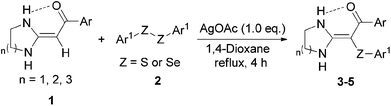
Initially, we screened the optimum conditions for the coupling reaction to synthesize 1-phenyl-2-(phenylthio)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethan-1-one 4a using HKA 1g and 1,2-di-phenyl disulfane 2a as the model substrates (Table 1). No reaction occurred without the addition of a catalyst at room temperature or refluxing temperature even after 24 hours (Table 1, entries 1 and 2). However, when AgOAc (1.0 mol%) was added to refluxed 1,4-dioxane, the yield of 4a reached 95% (Table 1, entries 3 and 4). Subsequently, trials with PdCl2, as well as other basic (t-BuOK, Cs2CO3, K2CO3, piperidine, and Et3N) or acidic (p-MBSA, TFA, and HAc) catalysts were screened, but the results demonstrated that only PdCl2 could slightly promote the reaction (Table 1, entries 5–13). We also attempted to change the AgOAc loading to 1.3 mol%, 0.7 mol%, 0.5 mol%, and 0.3 mol%, but the yields of 4a were nearly the same as that with 1.0 mol%, or even sacrificed. Therefore, the results showed that AgOAc (1.0 mol%) was the best (Table 1, entries 14–17). Further screening of solvents (toluene, DMF, EtOH, and CH3CN) indicated that the prospective product could be obtained with the highest yield (91%) (Table 1, entries 18–21) in toluene, but for the purposes of concise post-treatment and higher yield, 1,4-dioxane remained the most suitable reaction medium. Encouraged by these results, other silver salts, including AgNO3, AgCO3 and AgOTf, were checked (Table 1, entries 22–24). Hence, the optimum conditions included HKAs 1 (1.0 mol) and diaryl dichalcogenides 2 (1.0 mol) in 1,4-dioxane (10 mL) at reflux temperature for 4 hours with AgOAc (1.0 mol%) as the catalyst (Table 1, entries 4).
Table 1 Optimization of the reaction conditionsa

|
| Entry |
Cat. [concn/mol%] |
Solvent |
t (°C) |
Yieldb (%) |
| Reactions were carried out using 1g (1.0 mmol), 2a (1.0 mmol), catalyst, and solvent (10 mL) for 4 hours. Isolated yield, N. R. = no reaction. Prolonged the duration of the reaction to 24 hours. |
| 1c |
— |
1,4-Dioxane |
r. t. |
N. R. |
| 2c |
— |
1,4-Dioxane |
Reflux |
N. R. |
| 3 |
AgOAc (1.0) |
1,4-Dioxane |
r. t. |
32 |
| 4 |
AgOAc (1.0) |
1,4-Dioxane |
Reflux |
95 |
| 5 |
PdCl2 (1.0) |
1,4-Dioxane |
Reflux |
61 |
| 6 |
t-BuOK (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 7 |
Cs2CO3 (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 8 |
K2CO3 (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 9 |
Piperidine (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 10 |
Et3N (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 11 |
p-MBSA (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 12 |
TFA (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 13 |
HAc (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 14 |
AgOAc (1.3) |
1,4-Dioxane |
Reflux |
95 |
| 15 |
AgOAc (0.7) |
1,4-Dioxane |
Reflux |
87 |
| 16 |
AgOAc (0.5) |
1,4-Dioxane |
Reflux |
79 |
| 17 |
AgOAc (0.3) |
1,4-Dioxane |
Reflux |
68 |
| 18 |
AgOAc (1.0) |
Toluene |
Reflux |
91 |
| 19 |
AgOAc (1.0) |
DMF |
Reflux |
77 |
| 20 |
AgOAc (1.0) |
EtOH |
Reflux |
72 |
| 21 |
AgOAc (1.0) |
CH3CN |
Reflux |
51 |
| 22 |
AgNO3 (1.0) |
1,4-Dioxane |
Reflux |
N. R. |
| 23 |
Ag2CO3 (1.0) |
1,4-Dioxane |
Reflux |
90 |
| 24 |
AgOTf (0.5) |
1,4-Dioxane |
Reflux |
93 |
With the optimized reaction conditions in hand, we focused on the evaluation of the substrate scope (Table 2). A wide range of substituted HKAs 1a–p with three distinctively sized diazaheterocycles were employed to react with diaryl disulfides 2a–f or diphenyl diselenide 2g separately under the optimized conditions to afford the corresponding α-arylthio or α-phenylselanyl HKAs 3–5 in good yields. At first, we examined the scope of substrates 1. It was observed that the substituent group (Ar) on the HKAs had a certain influence on the yields. The system with an electron-donating group (OMe or Me) on the aryl ring reacted faster and gave higher yields than those with no substituent (H) or an electron-withdrawing group (F or Cl) (e.g. Table 2, entries 1–6). Notably, the reaction involving substrates with ortho-Cl always showed the lowest yield, which could be attributed to the electronic effect and steric hindrance (Table 2, entries 6, 22, and 33). On the other hand, as the diazaheterocycle is far away from the reactive α-position, the size of it (n) almost has no distinct effect on the yields of target materials (e.g. Table 2, entries 1, 17, and 34). Moreover, further exploration of the substrate scope referred to diaryl dichalcogenides 2 that mainly examined the impact of hetero-atoms (Z) and the substituent group (Ar1) on the yields of target molecules. Obviously, compounds 2, which have an electronegative element (S), gave higher yields than the relatively electropositive one (Se) (e.g. Table 2, entries 1 and 12). The substituents on the diaryl disulfides 2a–f exhibited trend in terms of the yields of target products, i.e., p-OMe ≈ p-Me > p-H > p-Cl > m-F > m-diCl (e.g. Table 2, entries 17 and 23–27). Similarly, the influence of substrates 2 can be explained by the electronic and the steric effect. In brief, these results confirmed that the substituent groups (Ar and Ar1) and the heteroatoms (Z) were the most significant influencing factors for this type of direct coupling reaction, which can be used to synthesize α-arylthio or α-phenylselanyl HKAs 3–5.
Table 2 Substrate scope of silver(I)-mediated coupling reaction of HKAs 1a–p with diaryl dichalcogenides 2a–ga
|
| Entry |
1 (n/Ar) |
2 (Z/Ar1) |
Product |
Yieldb (%) |
| Reaction conditions: 1 (1.0 mmol) and 2 (1.0 mmol) were added in 1,4-dioxane (10 mL) in the presence of AgOAc (1.0 equiv.), and then refluxed in a round-bottom flask for 4 hours. Isolated yield. |
| 1 |
1a (1/Ph) |
2a (S/Ph) |
3a |
94 |
| 2 |
1b (1/p-OMePh) |
2a (S/Ph) |
3b |
97 |
| 3 |
1c (1/p-MePh) |
2a (S/Ph) |
3c |
94 |
| 4 |
1d (1/p-FPh) |
2a (S/Ph) |
3d |
93 |
| 5 |
1e (1/p-ClPh) |
2a (S/Ph) |
3e |
89 |
| 6 |
1f (1/o-ClPh) |
2a (S/Ph) |
3f |
85 |
| 7 |
1a (1/Ph) |
2b (S/p-OMePh) |
3g |
94 |
| 8 |
1a (1/Ph) |
2c (S/p-MePh) |
3h |
95 |
| 9 |
1a (1/Ph) |
2d (S/p-ClPh) |
3i |
90 |
| 10 |
1a (1/Ph) |
2e (S/m-diClPh) |
3j |
84 |
| 11 |
1a (1/Ph) |
2f (S/m-FPh) |
3k |
88 |
| 12 |
1a (1/Ph) |
2g (Se/Ph) |
3l |
91 |
| 13 |
1b (1/p-OMePh) |
2g (Se/Ph) |
3m |
93 |
| 14 |
1c (1/p-MePh) |
2g (Se/Ph) |
3n |
91 |
| 15 |
1d (1/p-FPh) |
2g (Se/Ph) |
3o |
87 |
| 16 |
1e (1/p-ClPh) |
2g (Se/Ph) |
3p |
88 |
| 17 |
1g (2/Ph) |
2a (S/Ph) |
4a |
95 |
| 18 |
1h (2/p-OMePh) |
2a (S/Ph) |
4b |
98 |
| 19 |
1i (2/p-MePh) |
2a (S/Ph) |
4c |
96 |
| 20 |
1j (2/p-FPh) |
2a (S/Ph) |
4d |
92 |
| 21 |
1k (2/p-ClPh) |
2a (S/Ph) |
4e |
90 |
| 22 |
1l (2/o-ClPh) |
2a (S/Ph) |
4f |
87 |
| 23 |
1g (2/Ph) |
2b (S/p-OMePh) |
4g |
95 |
| 24 |
1g (2/Ph) |
2c (S/p-MePh) |
4h |
96 |
| 25 |
1g (2/Ph) |
2d (S/p-ClPh) |
4i |
93 |
| 26 |
1g (2/Ph) |
2e (S/m-diClPh) |
4j |
85 |
| 27 |
1g (2/Ph) |
2f (S/m-FPh) |
4k |
92 |
| 28 |
1g (2/Ph) |
2g (Se/Ph) |
4l |
92 |
| 29 |
1h (2/p-OMePh) |
2g (Se/Ph) |
4m |
95 |
| 30 |
1i (2/p-MePh) |
2g (Se/Ph) |
4n |
94 |
| 31 |
1j (2/p-FPh) |
2g (Se/Ph) |
4o |
90 |
| 32 |
1k (2/p-ClPh) |
2g (Se/Ph) |
4p |
86 |
| 33 |
1l (2/o-ClPh) |
2g (Se/Ph) |
4q |
83 |
| 34 |
1m (3/Ph) |
2a (S/Ph) |
5a |
92 |
| 35 |
1n (3/p-MePh) |
2a (S/Ph) |
5b |
93 |
| 36 |
1o (3/p-FPh) |
2a (S/Ph) |
5c |
90 |
| 37 |
1p (3/p-ClPh) |
2a (S/Ph) |
5d |
87 |
| 38 |
1m (3/Ph) |
2b (S/p-OMePh) |
5e |
96 |
| 39 |
1m (3/Ph) |
2c (S/p-MePh) |
5f |
95 |
| 40 |
1m (3/Ph) |
2d (S/p-ClPh) |
5g |
91 |
| 41 |
1m (3/Ph) |
2e (S/m-diClPh) |
5h |
83 |
| 42 |
1m (3/Ph) |
2f (S/m-FPh) |
5i |
88 |
| 43 |
1m (3/Ph) |
2g (Se/Ph) |
5j |
90 |
| 44 |
1n (3/p-MePh) |
2g (Se/Ph) |
5k |
93 |
| 45 |
1o (3/p-FPh) |
2g (Se/Ph) |
5l |
86 |
| 46 |
1p (3/p-ClPh) |
2g (Se/Ph) |
5m |
85 |
Finally, the scope of this synthetic protocol was evaluated for other HKAs (1q–r). The results are shown in Scheme 1 and demonstrate that 1q–r are also good substitutes in this synthetic procedure.
 |
| | Scheme 1 Preparation of α-arylthio-substituted HKAs. | |
It is also worth noting that the purification for all of the target products only required recrystallization rather than column chromatography. This easy post-treatment protocol makes this methodology facile, practical, and rapid to execute.
All compounds were characterized by IR, 1H NMR, 13C NMR spectroscopies as well as HRMS. The outcome of the regioselective direct coupling reaction was confirmed by X-ray diffraction analysis of selected single crystal of 3g (Fig. 2).
 |
| | Fig. 2 ORTEP view of the molecular structure of 3g (thermal ellipsoids are drawn at 30% probability). | |
In combination with known facts,25 a plausible mechanism for this reaction is depicted in Scheme 2. Initially, the Ag+ derived from AgOAc coordinates with equivalent chalcogens of diaryl dichalcogenides 2 to give 6. Subsequently, the coupling reaction of the α-carbon of HKAs 1 with the non-complexed heteroatom of 6 contributes to C-Z (S or Se) bond formation and affords target materials 3–5 and 7. The outcome of the coupling reaction is the direct synthesis of a sp2 hybrid carbon-chalcogen bond in situ.
 |
| | Scheme 2 Plausible mechanism for the synthesis of target materials 3–5. | |
Conclusions
In conclusion, we have developed the first protocol for the direct coupling reaction of HKAs with diaryl dichalcogenides in a remarkably mild, facile, and efficient way that covers a wide range of functionalized substrates, thus allowing in situ transformation of a variety of HKAs to α-arylthio and α-phenylselanyl target compounds in excellent yields with high regioselectivity. The developed method can be applied to sp2 hybrid carbon–chalcogen bond formation reactions in several other olefin systems. We are currently exploring the biological activity of the recently generated compounds as well as their potential application in the synthesis of novel heterocycles.
Experimental section
General information
All compounds were fully characterized by spectroscopic data. The NMR spectra were recorded on a Bruker DRX500 (1H: 500 MHz, 13C: 125 MHz) or DRX400 (1H: 400 MHz, 13C: 100 MHz); chemical shifts (δ) are expressed in ppm, J values are given in Hz, and deuterated CDCl3 was used as the solvent. IR spectra were recorded on a FT-IR Thermo Nicolet Avatar 360 using KBr pellets. The reactions were monitored by thin layer chromatography (TLC) using silica gel GF254. The melting points were determined on an XT-4A melting point apparatus and are uncorrected. HRMS was performed on an Agilent LC/Msd TOF instrument. All chemicals and solvents were used as received without further purification unless otherwise stated. The raw material 1 was synthesised according to the literature.26 The materials 3a–g were purchased from Aldrich Corporation Limited.
General procedure for the synthesis of 3–5
To a 25 mL round-bottomed flask, HKAs 1 (1 mmol), 1,2-diphenyldiselane 2 (1 mmol), AgOAc (1 mmol) in dioxane (15 mL) were added. The resulting mixture was heated to reflux and stirred until all starting diphenyldiselane was consumed, as monitored by TLC. After cooling to room temperature, the solid AgOAc was removed by filtration. The organic layer was then concentrated under reduced pressure to yield crude product. The crude compound was then purified by recrystallization with ethyl acetate and petroleum ether to afford compounds 3–5 as a white solid.
2-(Imidazolidin-2-ylidene)-1-phenyl-2-(phenylthio)ethanone (3a). White solid, yield 94%; mp 202–204 °C; IR (KBr): 3194, 1573, 1482, 1373, 1299, 735 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.53 (t, J = 8.8 Hz, 2H, NCH2), 3.88 (t, J = 8.8 Hz, 2H, NCH2), 5.78 (br, 1H, NH), 7.06–7.39 (m, ArH, 10H), 9.91 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 76.1, 124.7, 125.0, 127.4, 127.7, 129.1, 129.3, 141.0, 142.6, 168.7, 194.1; HRMS (TOF ES+): m/z calcd for C17H16N2NaOS [(M + Na+)], 319.0876; found, 319.0874.
2-(Imidazolidin-2-ylidene)-1-(4-methoxyphenyl)-2-(phenylthio)ethanone (3b). White solid, yield 97%; mp 176–177 °C; IR (KBr): 3366, 3269, 1576, 1369, 1241, 732 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.57 (t, J = 8.9 Hz, 2H, NCH2), 3.75 (s, 3H, CH3), 3.90 (t, J = 8.9 Hz, 2H, NCH2), 5.72 (br, 1H, NH), 6.74 (t, J = 8.6 Hz, 2H, ArH), 7.06–7.26 (m, 5H, ArH), 7.45 (t, J = 8.6 Hz, 2H, ArH), 9.95 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 55.6, 75.6, 112.9, 124.6, 125.0, 129.3, 129.6, 134.9, 141.0, 160.6, 168.9, 193.2; HRMS (TOF ES+): m/z calcd for C18H18N2NaO2S [(M + Na+)], 349.0981; found, 349.0971.
2-(Imidazolidin-2-ylidene)-2-(phenylthio)-1-(p-tolyl)ethanone (3c). White solid, yield 94%; mp 213–214 °C; IR (KBr): 3416, 3285, 1579, 1531, 1365, 1298, 744 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.28 (s, 3H, CH3), 3.57 (t, J = 8.8 Hz, 2H, NCH2), 3.91 (t, J = 8.8 Hz, 2H, NCH2), 5.72 (br, 1H, NH), 7.02 (d, J = 7.8 Hz, 2H, ArH), 7.06–7.33 (m, ArH, 7H), 9.94 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 21.8, 42.6, 45.8, 75.9, 124.7, 124.9, 127.5, 128.4, 129.3, 139.1, 139.7, 141.0, 168.7, 194.1; HRMS (TOF ES+): m/z calcd for C18H18N2NaOS [(M + Na+)], 333.1032; found, 333.1027.
1-(4-Fluorophenyl)-2-(imidazolidin-2-ylidene)-2-(phenylthio)ethanone (3d). White solid, yield 93%; mp 182–183 °C; IR (KBr): 3305, 1580, 1532, 1370, 1303, 743 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.50–3.57 (m, 2H, NCH2), 3.85–3.89 (m, 2H, NCH2), 5.80 (br, 1H, NH), 6.84–7.41 (m, 9H, ArH), 9.86 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 75.9, 114.5 (d, J = 20.0 Hz), 124.6, 125.1, 129.4, 129.7, 138.6, 140.7, 163.4 (d, J = 245.0 Hz), 168.7, 192.7; HRMS (TOF ES+): m/z calcd for C17H15FN2NaOS [(M + Na+)], 337.0781; found, 337.0783.
1-(4-Chlorophenyl)-2-(imidazolidin-2-ylidene)-2-(phenylthio)ethanone (3e). White solid, yield 89%; mp 200–202 °C; IR (KBr): 3413, 3267, 1579, 1532, 1370, 1306, 742 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.56 (t, J = 8.9 Hz, 2H, NCH2), 3.90 (t, J = 8.9 Hz, 2H, NCH2), 5.80 (br, 1H, NH), 7.06–7.34 (m, 9H, ArH), 9.85 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 76.0, 124.6, 125.2, 127.9, 129.0, 129.4, 134.9, 140.6, 140.9, 168.7, 192.6; HRMS (TOF ES+): m/z calcd for C17H15ClN2NaOS [(M + Na+)], 353.0486; found, 353.0486.
1-(2-Chlorophenyl)-2-(imidazolidin-2-ylidene)-2-(phenylthio)ethanone (3f). White solid, yield 85%; mp 194–195 °C; IR (KBr): 3193, 1582, 1540, 1482, 1381, 1299, 747 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.58 (t, J = 8.9 Hz, 2H, NCH2), 3.92 (t, J = 8.9 Hz, 2H, NCH2), 5.80 (br, 1H, NH), 7.05–7.30 (m, 9H, ArH), 9.74 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.5, 45.7, 125.1, 126.3, 127.6, 129.0, 129.5, 130.6, 140.2, 142.2, 168.0, 192.1; HRMS (TOF ES+): m/z calcd for C17H15ClN2NaOS [(M + Na+)], 353.0486; found, 353.0485.
2-(Imidazolidin-2-ylidene)-2-((4-methoxyphenyl)thio)-1-phenylethanone (3g). White solid, yield 94%; mp 218–220 °C; IR (KBr): 3403, 3304, 1589, 1533, 1482, 1367, 1298, 700 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.55 (t, J = 8.9 Hz, 2H, NCH2), 3.70 (s, 3H, CH3), 3.88 (t, J = 8.9 Hz, 2H, NCH2), 5.82 (br, 1H, NH), 6.78–7.40 (m, 9H, ArH), 9.87 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 55.8, 115.1, 126.4, 127.1, 127.4, 127.7, 129.1, 131.6, 142.7, 157.9, 168.8, 194.1; HRMS (TOF ES+): m/z calcd for C18H19N2O2S [(M + H+)], 327.1162; found, 327.1168.
2-(Imidazolidin-2-ylidene)-1-phenyl-2-(p-tolylselanyl)ethanone (3h). White solid, yield 95%; mp 196–197 °C; IR (KBr): 3424, 3318, 1578, 1359, 1298, 702 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.29 (s, 3H, ArCH3), 3.49–3.53 (m, 2H, NCH2), 3.85–3.88 (m, 2H, NCH2), 5.77 (br, 1H, NH), 7.01–7.41 (m, 9H, ArH), 9.91 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 21.3, 42.6, 45.8, 76.4, 124.8, 127.4, 127.7, 129.1, 130.1, 134.7, 137.4, 142.7, 168.7, 194.0; HRMS (TOF ES+): m/z calcd for C18H19N2OS [(M + H+)], 311.1213; found, 311.1216.
2-((4-Chlorophenyl)selanyl)-2-(imidazolidin-2-ylidene)-1-phenylethanone (3i). White solid, yield 90%; mp 211–213 °C; IR (KBr): 3200, 1581, 1377, 1299, 705 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.58 (t, J = 8.7 Hz, 2H, NCH2), 3.91 (t, J = 8.7 Hz, 2H, NCH2), 5.73 (br, 1H, NH), 7.03–7.37 (m, 9H, ArH), 9.90 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 75.8, 126.0, 127.3, 127.7, 129.2, 129.3, 130.7, 139.6, 142.4, 168.6, 194.2; HRMS (TOF ES+): m/z calcd for C17H16ClN2OS [(M + H+)], 331.0666; found, 331.0663.
2-((3,5-Dichlorophenyl)selanyl)-2-(imidazolidin-2-ylidene)-1-phenylethanone (3j). White solid, yield 84%; mp 213–214 °C; IR (KBr): 3208, 1575, 1377, 1300, 793 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.55–3.61 (m, 2H, NCH2), 3.89–3.94 (m, 2H, NCH2), 5.76 (br, 1H, NH), 6.95–7.34 (m, 8H, ArH), 9.89 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 74.7, 122.7, 125.2, 127.2, 127.8, 129.3, 135.8, 142.2, 145.3, 168.3, 194.1; HRMS (TOF ES+): m/z calcd for C17H15Cl2N2OS [(M + H+)], 365.0277; found, 365.0258.
2-((3-Fluorophenyl)selanyl)-2-(imidazolidin-2-ylidene)-1-phenylethanone (3k). White solid, yield 88%; mp 204–205 °C; IR (KBr): 3423, 3305, 1581, 1367, 1299, 733 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.53 (t, J = 8.8 Hz, 2H, NCH2), 3.88 (t, J = 8.8 Hz, 2H, NCH2), 5.79 (br, 1H, NH), 6.73–7.36 (m, 9H, ArH), 9.89 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 45.8, 75.5, 111.5 (d, J = 23.7 Hz), 111.9 (d, J = 21.3 Hz), 112.0, 120.3, 127.3, 127.8, 129.2, 130.6, 144.0, 163.2 (d, J = 245.0 Hz), 168.5, 194.1; HRMS (TOF ES+): m/z calcd for C17H16FN2OS [(M + H+)], 315.0962; found, 315.0965.
2-(Imidazolidin-2-ylidene)-1-phenyl-2-(phenylselanyl)ethanone (3l). White solid, yield 91%; mp 161–163 °C; IR (KBr): 3409, 3310, 1573, 1368, 1297, 728 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.48–3.52 (m, 2H, NCH2), 3.86–3.90 (m, 2H, NCH2), 5.78 (br, 1H, NH), 7.11–7.34 (m, 10H, ArH), 9.98 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.5, 46.0, 73.9, 125.8, 127.3, 127.5, 127.6, 128.9, 129.5, 135.7, 143.7, 168.5, 194.2; HRMS (TOF ES+): m/z calcd for C17H16N2NaOSe [(M + Na+)], 367.0320; found, 367.0315.
2-(Imidazolidin-2-ylidene)-1-(4-methoxyphenyl)-2-(phenylselanyl)ethanone (3m). White solid, yield 93%; mp 144–146 °C; IR (KBr): 3309, 1603, 1347, 818, 737 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.57–3.61 (m, 2H, NCH2), 3.78 (s, 3H, OCH3), 3.93–3.96 (m, 2H, NCH2), 5.77 (br, 1H, NH), 6.75–7.43 (m, 9H, ArH), 10.04 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 46.0, 55.6, 73.5, 112.9, 125.8, 127.4, 129.5, 129.6, 135.8, 135.9, 160.5, 168.7, 193.5; HRMS (TOF ES+): m/z calcd for C18H19N2O2Se [(M + H+)] 375.0606; found, 375.0601.
2-(Imidazolidin-2-ylidene)-2-(phenylselanyl)-1-(p-tolyl)-ethanone (3n). White solid, yield 91%; mp: 205–207 °C; IR (KBr): 3391, 3252, 1572, 1529, 1366, 1300, 733 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.12 (s, ArCH3), 3.55–3.66 (m, 2H, NCH2), 3.88–3.95 (m, 2H, NCH2), 5.74 (br, 1H, NH), 6.74 (d, J = 8.6 Hz, 2H, ArH), 7.12–7.27 (m, 5H, ArH), 7.39 (d, J = 8.6 Hz, 2H, ArH), 10.02 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.6, 46.0, 55.6, 67.5, 112.8, 125.8, 127.4, 129.5, 129.6, 135.8, 135.9, 160.5, 168.7, 193.5; HRMS (TOF ES+): m/z calcd for C18H19Na2OSe [(M + H+)], 359.0657; found, 359.0661.
1-(4-Fluorophenyl)-2-(imidazolidin-2-ylidene)-2-(phenylselanyl)ethanone (3o). White solid, yield 87%; mp 167–169 °C; IR (KBr): 3412, 3312, 1575, 1525, 1367, 1298, 726 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.53–3.58 (m, 2H, NCH2), 3.90–3.94 (m, 2H, NCH2), 5.81 (br, 1H, NH), 6.85–6.90 (m, 2H, ArH), 7.14–7.15 (m, 1H, ArH), 7.22–7.37 (m, 6H, ArH), 9.96 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.5, 46.0, 73.7, 114.5 (d, J = 20.0 Hz), 126.0, 127.4, 129.6, 129.6, 135.5, 139.6, 163.2 (d, J = 246.3 Hz), 168.6, 192.9; HRMS (TOF ES+): m/z calcd for C17H16FN2OSe [(M + H+)], 363.0406; found, 363.0412.
1-(4-Chlorophenyl)-2-(imidazolidin-2-ylidene)-2-(phenylselanyl)ethanone (3p). White solid, yield 88%; mp 184–186 °C; IR (KBr): 3405, 3263, 1576, 1528, 1369, 1305, 736 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.56–3.60 (m, 2H, NCH2), 3.91–3.96 (m, 2H, NCH2), 5.77 (br, 1H, NH), 7.13–7.28 (m, 9H, ArH), 9.95 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 42.5, 46.0, 73.7, 126.0, 127.3, 127.8, 128.9, 129.6, 134.7, 135.4, 141.9, 168.6, 192.8; HRMS (TOF ES+): m/z calcd for C17H16ClN2OSe [(M + H+)], 379.0111; found, 379.0103.
2-(Nitro(phenylthio)methylene)imidazolidine (3q). White solid, yield 88%; mp 163.5–164 °C; IR (KBr): 3350, 3256, 1576, 1392, 1335, 738 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.75–3.79 (m, 2H, NCH2), 3.95–4.00 (m, 2H, NCH2), 5.83 (br, 1H, NH), 7.13–7.17 (m, 3H, ArH), 7.24–7.28 (m, 2H, ArH), 8.83 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 43.1, 45.6, 98.4, 125.6, 125.9, 129.1, 136.1, 163.3; HRMS (TOF ES+): m/z calcd for C10H11N3NaO2S [(M + Na+)], 260.0464; found, 260.0462.
1-Phenyl-2-(phenylthio)-2-(tetrahydropyrimdin-2(1H)-ylidene)ethanone (4a). White solid, yield 95%; mp 167–169 °C; IR (KBr): 3352, 3279, 1586, 1344, 1205, 742 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.79–1.82 (m, 2H, NCH2), 3.27–3.43 (m, 4H, NCH2), 6.49 (br, 1H, NH), 7.04–7.08 (m, 3H, ArH), 7.15–7.18 (m, 4H, ArH), 7.24–7.28 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 38.8, 39.5, 78.4, 124.7, 125.0, 126.8, 127.7, 128.5, 129.2, 141.0, 143.9, 160.8, 193.0; HRMS (TOF ES+): m/z calcd for C18H19N2OS [(M + H+)], 311.1213; found, 211.1209.
1-(4-Methoxyphenyl)-2-(phenylthio)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4b). White solid, yield 98%; mp 177–178 °C; IR (KBr): 3364, 3050, 1585, 1344, 1241, 735 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.93–1.96 (m, 2H, CH2), 3.30–3.35 (m, 2H, NCH2), 3.43–3.49 (m, 2H, NCH2), 3.74 (s, 3H, OCH3), 6.49 (br, 1H, NH), 6.71 (d, J = 7.3 Hz, 2H, ArH), 7.08–7.26 (m, 5H, ArH), 7.33 (d, J = 7.3 Hz, 2H, ArH), 12.05 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 38.8, 39.5, 55.5, 112.9, 124.6, 125.0, 128.9, 129.3, 136.3, 141.1, 160.0, 160.9, 192.4; HRMS (TOF ES+): m/z calcd for C19H21N2O2S [(M + H+)], 341.1318; found, 341.1316.
2-(Phenylthio)-2-(tetrahydropyrimidin-2(1H)-ylidene)-1-(p-tolyl)ethanone (4c). White solid, yield 96%; mp 175–177 °C; IR (KBr): 3330, 3052, 1584, 1339, 1164, 746 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.88–1.92 (m, 2H, NCH2), 2.26 (s, 3H, ArCH3), 3.25–3.29 (m, 2H, NCH2), 3.40–3.47 (m, 2H, NCH2), 6.48 (br, 1H, NH), 6.98 (d, J = 7.6 Hz, 2H, ArH), 6.97–7.12 (m, 3H, ArH), 7.22–7.25 (m, 4H, ArH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 21.8, 38.8, 39.5, 78.2, 124.6, 125.0, 127.0, 128.3, 129.2, 138.3, 141.1, 160.8, 193.0; HRMS (TOF ES+): m/z calcd for C19H21N2OS, [(M + H+)], 325.1369; found, 325.1365.
1-(4-Fluorophenyl)-2-(phenylthio)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4d). White solid, yield 92%; mp 122–124 °C; IR (KBr): 3322, 3060, 1586, 1343, 1210, 746 cm−1; 1H NMR (400 MHz, CDCl3): δ = 1.93–1.99 (m, 2H, CH2), 3.39–3.43 (m, 4H, NCH2), 6.51 (br, 1H, NH), 6.83–7.32 (m, 9H, ArH), 11.92 (br, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 20.0, 39.0, 39.0, 77.9, 114.1 (d, J = 26.3 Hz), 124.2, 124.7, 128.6 (d, J = 8.0 Hz), 128.9, 139.4, 140.3, 160.4, 162.5 (d, J = 245.0 Hz), 191.3; HRMS (TOF ES+): m/z calcd for C18H17FN2NaOS [(M + Na+)], 351.0938; found, 351.0637.
1-(4-Chlorophenyl)-2-(phenylthio)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4e). White solid, yield 90%; mp 180–181 °C; IR (KBr): 3330, 3052, 1584, 1339, 1164, 746 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.95–1.98 (m, 2H, CH2), 3.39–3.44 (m, 4H, NCH2), 6.52 (br, 1H, NH), 7.07–7.48 (m, 9H, ArH), 11.85 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 39.1, 39.1, 78.5, 124.6, 125.2, 127.9, 128.5, 129.3, 134.2, 140.6, 142.2, 160.8, 191.6; HRMS (TOF ES+): m/z calcd for C18H17ClN2NaOS [(M + Na+)], 367.0642; found, 367.0637.
1-(2-Chlorophenyl)-2-(phenylthio)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4f). White solid, yield 87%; mp 163–165 °C; IR (KBr): 3261, 1593, 1341, 1210, 747 cm−1; 1H NMR (400 MHz, CDCl3): δ = 1.95–1.99 (m, 2H, CH2), 3.38–3.44 (m, 4H, NCH2), 7.00–7.28 (m, 9H, ArH); 13C NMR (100 MHz, CDCl3): δ = 19.9, 38.7, 38.7, 79.5, 124.5, 124.7, 125.9, 126.9, 128.4, 128.6, 129.1, 130.1, 139.7, 142.4, 160.0, 189.9; HRMS (TOF ES+): m/z calcd for C18H17ClN2NaOS [(M + Na+)], 367.0642; found, 367.0635.
2-((4-Methoxyphenyl)selanyl)-1-phenyl-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4g). White solid, yield 95%; mp 174–176 °C; IR (KBr): 3368, 3187, 1592, 1354, 1232, 743 cm−1; 1H NMR (400 MHz, CDCl3): δ = 1.93–1.96 (m, 2H, CH2), 3.35–3.45 (m, 4H, NCH2), 3.76 (s, 3H, OCH3), 6.56 (br, 1H, NH), 6.78–7.31 (m, 9H, ArH), 11.93 (br, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 20.1, 38.9, 38.9, 55.5, 79.2, 114.7, 125.8, 126.6, 127.3, 128.1, 131.4, 143.6, 157.5, 160.5, 192.6; HRMS (TOF ES+): m/z calcd for C19H21N2O2S [(M + H+)], 341.1318; found, 341.1319.
1-Phenyl-2-(tetrahydropyrimidin-2(1H)-ylidene)-2-(p-tolylselanyl)ethanone (4h). White solid, yield 96%; mp 213–214 °C; IR (KBr): 3369, 1593, 1342, 1208, 800 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.94–1.97 (m, 2H, CH2), 2.29 (s, 3H, ArCH3), 3.31–3.34 (m, 2H, NCH2), 3.45–3.49 (m, 2H, NCH2), 6.51 (br, 1H, NH), 6.99–7.32 (m, 9H, ArH), 11.97 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 21.3, 38.8, 39.5, 78.7, 124.7, 126.9, 127.6, 128.4, 130.0, 134.7, 137.4, 143.9, 160.8, 193.0; HRMS (TOF ES+): m/z calcd for C19H21N2OS [(M + H+)], 325.1369; found, 325.1364.
2-((4-Chlorophenyl)selanyl)-1-phenyl-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4i). White solid, yield 93%; mp 210–211 °C; IR (KBr): 3360, 1588, 1344, 1210, 809 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.93–1.96 (m, 2H, CH2), 3.33–3.36 (m, 2H, NCH2), 3.44–3.47 (m, 2H, NCH2), 6.43 (br, 1H, NH), 7.00–7.27 (m, 9H, ArH), 11.89 (br, 1H, NH). 13C NMR (125 MHz, CDCl3): δ = 20.4, 38.8, 39.5, 78.1, 125.9, 126.7, 127.7, 128.6, 129.3, 130.7, 139.6, 143.7, 160.7, 193.1; HRMS (TOF ES+): m/z calcd for C18H18ClN2OS [(M + H+)], 345.0823; found, 345.0829.
2-((3,5-Dichlorophenyl)selanyl)-1-phenyl-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4j). White solid, yield 85%; mp 223–225 °C; IR (KBr): 3276, 3067, 1596, 1334, 1214, 786 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.96–2.01 (m, 2H, CH2), 3.34–3.38 (m, 2H, NCH2), 3.46–3.50 (m, 2H, NCH2), 6.35 (br, 1H, NH), 6.94–7.27 (m, 8H, ArH), 11.89 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.3, 38.8, 39.6, 122.7, 125.2, 126.7, 127.8, 128.7, 135.8, 143.5, 145.3, 160.5, 193.4; HRMS (TOF ES+): m/z calcd for C18H17Cl2N2OS [(M + H+)], 379.0433; found, 379.0437.
2-((4-Fluorophenyl)selanyl)-1-phenyl-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4k). White solid, yield 92%; mp 181–183 °C; IR (KBr): 3283, 1590, 1342, 1210, 780 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.95–1.98 (m, 2H, CH2), 3.32–3.36 (m, 2H, NCH2), 3.67–3.72 (m, 2H, NCH2), 6.43 (br, 1H, NH), 6.73–7.29 (m, 9H, ArH), 11.93 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 38.8, 39.5, 111.5 (d, J = 23.8 Hz), 112.0 (d, J = 21.3 Hz), 120.3, 123.1, 126.8, 127.7, 128.6, 130.5, 143.9, 160.7, 163.6 (d, J = 246.3 Hz), 164.8, 193.2; HRMS (TOF ES+): m/z calcd for C18H18FN2OS [(M + H+)], 329.1118; found, 329.1118.
1-Phenyl-2-(phenylselanyl)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4l). White solid, yield 92%; mp 162–164 °C; IR (KBr): 3353, 1587, 1343, 741 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.94–1.98 (m, 2H, CH2), 3.32–3.37 (m, 2H, NCH2), 3.46–3.50 (m, 2H, NCH2), 6.57 (br, 1H, NH), 7.15–7.31 (m, 10H, ArH), 12.12 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.0, 38.5, 39.2, 77.8, 125.3, 126.3, 126.8, 127.1, 127.7, 129.0, 135.3, 144.6, 160.1, 192.6; HRMS (TOF ES+): m/z calcd for C18H19N2OSe [(M + H+)], 359.0657; found, 359.0668.
1-(4-Methoxyphenyl)-2-(phenylselanyl)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4m). White solid, yield 95%; mp 154–156 °C; IR (KBr): 3353, 1581, 1344, 1240, 799 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.90–1.95 (m, 2H, CH2), 3.33–3.45 (m, 4H, NCH2), 3.73 (s, 3H, OCH3), 6.53 (br, 1H, NH), 6.69–7.29 (m, 9H, ArH); 13C NMR (125 MHz, CDCl3): δ = 20.5, 39.2, 39.8, 55.5, 112.8, 125.8, 127.3, 128.8, 129.5, 135.9, 137.6, 159.9, 160.8, 192.5; HRMS (TOF ES+): m/z calcd for C19H21N2O2Se [(M + H+)], 389.0763; found, 389.0768.
2-(Phenylselanyl)-2-(tetrahydropyrimidin-2(1H)-ylidene)-1-(p-tolyl)ethanone (4n). White solid, yield 94%; mp 157–159 °C; IR (KBr): 3363, 3325, 1582, 1341, 1207, 742 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.94–1.97 (m, 2H, CH2), 2.31 (s, 3H, ArCH3), 3.34–3.47 (m, 4H, NCH2), 6.56 (br, 1H, NH), 7.01–7.30 (m, 9H, ArH), 12.17 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.5, 21.8, 39.0, 39.7, 78.1, 125.8, 126.9, 127.3, 128.3, 129.5, 135.9, 138.0, 142.2, 160.7, 193.2; HRMS (TOF ES+): m/z calcd for C19H21N2OSe [(M + H+)], 373.0814; found, 373.0817.
1-(4-Fluorophenyl)-2-(phenylselanyl)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4o). White solid, yield 90%; mp 139–140 °C; IR (KBr): 3322, 1582, 1346, 1210, 740 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.93–1.98 (m, 2H, CH2), 3.40–3.47 (m, 4H, NCH2), 6.59 (br, 1H, NH), 6.85–7.30 (m, 9H, ArH), 12.07 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 39.6, 40.9, 78.5, 114.4 (d, J = 21.3 Hz), 125.9, 127.2, 128.9, 129.6, 135.6, 141.1, 160.7, 163.8, 191.8; HRMS (TOF ES+): m/z calcd for C18H18FN2OSe [(M + H+)], 377.0563; found, 377.0565.
1-(4-Chlorophenyl)-2-(phenylselanyl)-2-(tetrahydropyrimidin-2(1H)-ylidene)ethanone (4p). White solid, yield 86%; mp 176–177 °C; IR (KBr): 3319, 3057, 1581, 1345, 1205, 740 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.91–1.94 (m, 2H, CH2), 3.36–3.41 (m, 4H, NCH2), 6.58 (br, 1H, NH), 7.12–7.25 (m, 9H, ArH), 11.98 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 39.4, 39.4, 78.3, 126.0, 127.2, 127.8, 128.4, 129.6, 133.9, 135.5, 143.4, 160.6, 191.6; HRMS (TOF ES+): m/z calcd for C18H18ClN2OSe [(M + H+)], 393.0265; found, 393.0267.
1-(2-Chlorophenyl)-2-(imidazolidin-2-ylidene)-2-(phenylselanyl)ethanone (4q). White solid, yield 83%; mp 151–153 °C; IR (KBr): 3350, 1589, 1350, 1211, 747 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.89–1.93 (m, 2H, CH2), 3.28–3.32 (m, 2H, NCH2), 3.41–3.45 (m, 2H, NCH2), 6.49 (br, 1H, NH), 6.99–7.24 (m, 9H, ArH), 11.78 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 20.4, 39.0, 39.6, 79.8, 125.9, 126.4, 127.7, 128.7, 129.4, 130.5, 134.9, 144.0, 160.2, 190.1; HRMS (TOF ES+): m/z calcd for C18H17ClN2NaOSe [(M + Na+)], 415.0087; found, 415.0083.
2-(Nitro(phenylthio)methylene)hexahydropyrimidine (4r). White solid, yield 90%; mp 152.5–153 °C; IR (KBr): 3284, 1585, 1356, 1200, 1127, 738 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.93–1.98 (m, 2H, CH2), 3.42–3.47 (m, 4H, NCH2), 7.11–7.15 (m, 3H, ArH), 7.22–7.26 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 19.0, 39.0, 39.0, 100.8, 125.5, 125.9, 129.1, 135.7, 155.7; HRMS (TOF ES+): m/z calcd for C11H13N3NaO2S [(M + Na+)], 274.0621; found, 274.0619.
2-(1,3-Diazepan-2-ylidene)-1-phenyl-2-(phenylthio)ethanone (5a). White solid, yield 92%; mp 177–178 °C; IR (KBr): 3363, 1594, 1343, 1202, 742 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.75–1.81 (m, 4H, CH2CH2), 3.20–3.23 (m, 2H, NCH2), 3.45–3.48 (m, 2H, NCH2), 6.55 (br, 1H, NH), 7.08–7.31 (m, 10H, ArH), 12.14 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 28.0, 28.3, 45.5, 46.3, 81.6, 124.8, 125.1, 126.9, 127.6, 128.7, 129.3, 140.9, 143.9, 170.7, 194.2; HRMS (TOF ES+): m/z calcd for C19H21N2OS [(M + H+)], 325.1369; found, 325.1368.
2-(1,3-Diazepan-2-ylidene)-2-(phenylselanyl)-1-(p-tolyl)-ethanone (5b). White solid, yield 93%; mp 157–158 °C; IR (KBr): 3317, 2932, 1605, 1349, 1200, 741 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.73–1.80 (m, 4H, CH2CH2), 2.29 (s, 3H, ArCH3), 3.18–3.20 (m, 2H, NCH2), 3.43–3.47 (m, 2H, NCH2), 6.54 (br, 1H, NH), 7.01 (d, J = 7.7 Hz, 2H), 7.00–7.13 (m, 3H, ArH), 7.24–7.27 (m, 3H, ArH), 12.20 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 21.8, 28.1, 28.3, 45.5, 46.3, 81.4, 124.8, 125.1, 127.1, 128.3, 128.3, 129.3, 138.6, 141.0, 170.8, 194.2; HRMS (TOF ES+): m/z calcd for C20H23N2OS [(M + H+)], 339.1526; found, 339.1518.
2-(1,3-Diazepan-2-ylidene)-1-(4-fluorophenyl)-2-(phenylthio)ethanone (5c). White solid, yield 90%; mp 124–125 °C; IR (KBr): 3331, 3060, 1591, 1348, 1207, 847, 740 cm−1; 1H NMR (500 MHz, CDCl3); δ = 1.76–1.84 (m, 4H, CH2CH2), 3.22–3.26 (m, 2H, NCH2), 3.46–3.50 (m, 2H, NCH2), 6.59 (br, 1H, NH), 6.85–6.91 (m, 2H, ArH), 7.09–7.14 (m, 3H, ArH), 7.25–7.29 (m, ArH, 2H), 7.33–7.36 (m, ArH, 2H), 12.12 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 28.0, 28.2, 45.5, 46.3, 81.5, 114.4 (d, J = 21.3 Hz), 124.7, 125.2, 129.2, 129.4, 139.9, 140.6, 163.0 (d, J = 245.0 Hz), 170.7, 192.80; HRMS (TOF ES+): m/z calcd for C19H19FN2NaOS [(M + Na+)], 365.1094; found, 365.1087.
1-(4-Chlorophenyl)-2-(1,3-diazepan-2-ylidene)-2-(phenylthio)ethanone (5d). White solid, yield 87%; mp 156–157 °C; IR (KBr): 3325, 3053, 1594, 1349, 1202, 833, 744 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.68–1.81 (m, 4H, CH2CH2), 3.20–3.24 (m, 2H, NCH2), 3.67–3.70 (m, 2H, NCH2), 6.56 (br, 1H, NH), 7.05–7.25 (m, 9H, ArH), 12.06 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 27.9, 28.2, 45.5, 46.3, 81.5, 124.7, 125.3, 127.8, 128.5, 129.4, 134.4, 140.5, 142.2, 170.6, 192.7; HRMS (TOF ES+): m/z calcd for C19H20ClN2OS [(M + H+)], 359.0979; found, 359.0975.
2-(1,3-Diazepan-2-ylidene)-2-((4-methoxyphenyl)thio)-1-phenylethanone (5e). White solid, yield 96%; mp 171–172 °C; IR (KBr): 3316, 2930, 1600, 1342, 1238, 816 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.75–1.79 (m, 4H, CH2CH2), 3.19–3.23 (m, 2H, NCH2), 3.42–3.47 (m, 2H, NCH2), 3.76 (s, 3H, OCH3), 6.64 (br, 1H, NH), 6.80 (d, J = 8.5 Hz), 6.98 (d, J = 8.5 Hz), 7.18–7.26 (m, 3H, ArH), 7.28–7.32 (m, 2H, ArH), 12.11 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 28.0, 28.3, 45.5, 46.3, 55.8, 82.8, 115.1, 126.3, 126.9, 127.6, 128.6, 131.5, 144.0, 157.9, 170.8, 194.1; HRMS (TOF ES+): m/z calcd for C20H23N2O2S [(M + H+)], 355.1475; found, 355.147.
2-(1,3-Diazepan-2-ylidene)-1-phenyl-2-(p-tolylthio)ethanone (5f). White solid, yield 95%; mp 175–177 °C; IR (KBr): 3334, 3046, 1600, 1345, 1200, 800 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.75–1.81 (m, 4H, CH2CH2), 2.30 (s, 3H, ArCH3), 3.19–3.23 (m, 2H, NCH2), 3.42–3.48 (m, 2H, NCH2), 6.58 (br, 1H, NH), 6.98 (d, J = 8.2 Hz, 2H, ArH), 7.06 (d, J = 8.2 Hz, 2H, ArH), 7.17–7.32 (m, 5H, ArH), 12.14 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 21.3, 28.0, 28.3, 45.5, 46.3, 81.9, 124.8, 126.9, 127.6, 128.6, 130.1, 134.8, 137.2, 143.9, 170.7, 194.1; HRMS (TOF ES+): m/z calcd for C20H23N2OS [(M + H+)], 339.1526; found, 339.1527.
2-((4-Chlorophenyl)thio)-2-(1,3-diazepan-2-ylidene)-1-phenylethanone (5g). White solid, yield 91%; mp 200–202 °C; IR (KBr): 3318, 1603, 1346, 1199, 803 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.74–182 (m, 4H, CH2CH2), 3.21–3.23 (m, 2H, NCH2), 3.45–3.48 (m, 2H, NCH2), 6.48 (br, 1H, NH), 7.00 (d, J = 8.6 Hz, 2H, ArH), 7.17–7.28 (m, 7H, ArH), 12.1 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 27.9, 28.2, 45.5, 46.3, 81.2, 126.0, 126.7, 127.7, 128.8, 129.4, 130.8, 139.5, 143.7, 170.5, 194.2; HRMS (TOF ES+): m/z calcd for C19H20 ClN2OS [(M + H+)], 359.0979; found, 359.0982.
2-(1,3-Diazepan-2-ylidene)-2-((3,5-dichlorophenyl)thio)-1-phenylethanone (5h). White solid, yield 83%; mp 139–141 °C; IR (KBr): 3308, 1561, 1348, 1206, 791 cm−1; 1H NMR (500 MHz, CDCl3); δ = 1.79–1.84 (m, 4H, CH2CH2), 3.24–3.28 (m, 2H, NCH2), 3.47–3.50 (m, 2H, NCH2), 6.38 (br, 1H, NH), 6.91 (s, 2H, ArH), 7.04 (s, 1H, ArH), 7.20–7.27 (m, 5H, ArH), 12.07 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 27.8, 28.1, 45.4, 46.3, 80.0, 122.7, 125.3, 126.6, 127.8, 128.9, 135.8, 143.5, 145.1, 170.1, 194.3; HRMS (TOF ES+): m/z calcd for C19H19Cl2N2OS [(M + H+)], 393.0590; found, 393.0596.
2-(1,3-Diazepan-2-ylidene)-2-((4-fluorophenyl)thio)-1-phenylethanone (5i). White solid, yield 88%, mp 188–189 °C; IR (KBr): 3255, 1556, 1354, 1210, 776 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.75–1.83 (m, 4H, CH2CH2), 3.19–3.23 (m, 2H, NCH2), 3.42–3.47 (m, 2H, NCH2), 6.48 (br, 1H, NH), 6.74–6.86 (m, 3H, ArH), 7.15–7.83 (m, 6H, ArH), 12.09 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 27.9, 28.2, 45.5, 46.3, 111.5 (d, J = 23.8 Hz), 112.0 (d, J = 21.3 Hz), 120.4, 126.8, 127.3, 127.7, 128.7, 128.8, 130.6, 143.7, 163.8 (d, J = 246.3 Hz), 170.4, 194.2; HRMS (TOF ES+): m/z calcd for C19H20FN2OS [(M + H+)], 343.1275; found, 343.1271.
2-(1,3-Diazepan-2-ylidene)1-phenyl-2-(phenylselanyl)ethanone (5j). White solid, yield 90%; mp 154–156 °C; IR (KBr): 3347, 1593, 1342, 1202, 737 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.72–1.77 (m, 4H, CH2CH2), 3.15–3.19 (m, 2H, CH2), 3.42–3.46 (m, 2H, CH2), 6.53 (br, 1H, NH), 7.12–7.28 (m, 10H, ArH), 12.17 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 28.2, 28.2, 45.7, 46.4, 81.6, 125.9, 126.8, 127.5, 127.6, 128.5, 129.5, 135.7, 145.0, 170.7, 194.4; HRMS (TOF ES+): m/z calcd for C19H21N2OSe [(M + H+)], 373.0814; found, 373.0816.
2-(1,3-Diazepan-2-ylidene)-2-(phenylselanyl)-1-(p-tolyl)ethanone (5k). White solid, yield 93%, mp 125–127 °C; IR (KBr): 3308, 1603, 1346, 1204, 818, 736 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.72–1.77 (m, 4H, CH2CH2), 3.15–3.18 (m, 2H, NCH2), 3.41–3.45 (m, 2H, NCH2), 6.50 (br, 1H, NH), 7.00 (d, J = 7.4 Hz, 2H, ArH), 7.13–7.26 (m, 7H, ArH), 12.19 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 21.8, 28.1, 28.2, 45.7, 46.5, 81.5, 125.9, 127.0, 127.4, 128.3, 129.5, 135.8, 138.5, 142.1, 170.8, 194.5; HRMS (TOF ES+): m/z calcd for C20H23N2OSe [(M + H+)], 387.0970; found, 387.0970.
2-(1,3-Diazepan-2-ylidene)-1-(4-fluorophenyl)-2-(phenylselanyl)ethanone (5l). White solid, yield 86%; mp 137–138 °C; IR (KBr): 3319, 3053, 1598, 1349, 1207, 841, 737 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.73–1.80 (m, 4H, CH2CH2), 2.17 (s, 3H, ArH), 3.18–3.22 (m, 2H, NCH2), 3.43–3.48 (m, 2H, NCH2), 6.54 (br, 1H, NH), 6.86 (t, J = 8.5 Hz, 2H, ArH), 7.17–7.30 (m, 7H, ArH), 12.14 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 28.0, 28.2, 45.6, 46.5, 81.5, 114.4 (d, J = 21.3 Hz), 126.0, 127.4, 129.0, 129.6, 135.5, 141.0, 163.0 (d, J = 245.0 Hz), 170.7, 193.1; HRMS (TOF ES+): m/z calcd for C19H20FN2Ose [(M + H+)], 391.0719; found, 391.0718.
1-(4-Chlorophenyl)-2-(1,3-diazepan-2-ylidene)-2-(phenylselanyl)ethanone (5m). White solid, yield 85%; mp 141–143 °C; IR (KBr): 3315, 3064, 1590, 1346, 1203, 739 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.77–1.83 (m, 4H, CH2CH2), 3.22–3.25 (m, 2H, NCH2), 3.46–3.50 (m, 2H, NCH2), 6.58 (br, 1H, NH), 7.14–7.33 (m, 9H, ArH), 12.15 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 28.0, 28.0, 45.6, 46.4, 81.5, 126.1, 127.4, 127.8, 128.4, 129.6, 134.3, 135.4, 143.4, 170.6, 192.9; HRMS (TOF ES+): m/z calcd for C19H20ClN2OSe [(M + H+)], 407.0424; found, 407.0416.
Acknowledgements
This work was supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT13095), the National Natural Science Foundation of China (Nos. U1202221, 21262042, 21362042, 21162037, and 81160384) and the Reserve Talent Foundation of Yunnan Province (no. 2012HB001).
References
-
(a) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242–3272 RSC;
(b) J. F. Hartwig, Nature, 2008, 455, 314–322 CrossRef CAS PubMed;
(c) D. S. Glueck, Dalton Trans., 2008, 5276–5286 RSC;
(d) I. P. Beletskaya, Pure Appl. Chem., 2005, 77, 2021–2027 CrossRef CAS;
(e) S. L. Buchwald, Adv. Synth. Catal., 2004, 346, 1524 CrossRef CAS;
(f) J. F. Hartwig, Acc. Chem. Res., 1998, 31, 852–860 CrossRef CAS.
-
(a) I. P. Beletskaya and V. P. Ananikov, in Catalyzed Carbon-Heteroatom Bond Formation, ed. A. K. Yudin, Wiley-VCH, Weinheim, 1st edn, 2011, pp. 69–118 Search PubMed;
(b) Organoselenium Chemistry: Synthesis and Reactions, ed. T. Wirth, Wiley-VCH, Weinheim, 1st edn, 2012 Search PubMed.
- T. Nishimura, A. K. Unni, S. Yokoshima and T. Fukuyama, J. Am. Chem. Soc., 2013, 135, 3243–3247 CrossRef CAS PubMed.
-
(a) G. La Regina, R. Bai, W. M. Rensen, E. Di Cesare, A. Coluccia, F. Piscitelli, V. Famiglini, A. Reggio, M. Nalli, S. Pelliccia, E. Da Pozzo, B. Costa, I. Granata, A. Porta, B. Maresca, A. Soriani, M. L. Iannitto, A. Santoni, J.-J. Li, M. M. Cona, F. Chen, Y.-C. Ni, A. Brancale, G. Dondio, S. Vultaggio, M. Varasi, C. Mercurio, C. Martini, E. Hamel, P. Lavia, E. Novellino and R. Silvestr, J. Med. Chem., 2013, 56, 123–149 CrossRef CAS PubMed;
(b) B. Wellenzohn, U. Lessel, A. Beller, T. Isambert, C. Hoenke and B. Nosse, J. Med. Chem., 2012, 55, 11031–11041 CrossRef CAS PubMed;
(c) R. Puig-de-la-Bellacasa, L. Giménez, S. Pettersson, R. Pascual, E. Gonzalo, J. A. Esté, B. Clotet, J. I. Borrell, J. Teixidó, Eur. J. Med. Chem., 2012, 54, 159–174 Search PubMed;
(d) G. La Regina, R. Bai, W. Rensen, A. Coluccia, F. Piscitelli, V. Gatti, A. Bolognesi, A. Lavecchia, I. Granata, A. Porta, B. Maresca, A. Soriani, M. L. Iannitto, M. Mariani, A. Santoni, A. Brancale, C. Ferlini, G. Dondio, M. Varasi, C. Mercurio, E. Hamel, P. Lavia, E. Novellino and R. Silvestri, J. Med. Chem., 2011, 54, 8394–8406 CrossRef CAS PubMed;
(e) H. Sekiguchi, K. Muranaka, A. Osada, S. Ichikawa and A. Matsuda, Bioorg. Med. Chem., 2010, 18, 5732–5737 CrossRef CAS PubMed;
(f) J. H. M. Lange, M. A. W. van der Neut, A. J. M. Borst, M. Yildirim, H. H. van Stuivenberg, B. J. van Vliet and C. G. Kruse, Bioorg. Med. Chem. Lett., 2010, 20, 2770–2775 CrossRef CAS PubMed;
(g) Y. Leblanc, P. Roy, C. Dufresne, N. Lachance, Z.-Y. Wang, G. O'Neill, G. Greig, D. Denis, M.-C. Mathieu, D. Slipetz, N. Sawyer and N. Tsou, Bioorg. Med. Chem. Lett., 2009, 19, 2125–2128 CrossRef CAS PubMed;
(h) A. Gagnon, S. Landry, R. Coulombe, A. Jakalian, I. Guse, B. Thavonekham, P. R. Bonneau, C. Yoakim and B. Simoneau, Bioorg. Med. Chem. Lett., 2009, 19, 1199–1205 CrossRef CAS PubMed;
(i) N. Jarkas, R. J. Voll, L. Williams, J. R. Votaw, M. Owens and M. M. Goodman, J. Med. Chem., 2008, 51, 271–281 CrossRef CAS PubMed.
- R. W. Baker, H. Radzey, N. T. Lucas and P. Turner, Organometallics, 2012, 31, 5622–5633 CrossRef CAS.
- M. J. E. Resendiz, A. Schön, E. Freire and M. M. Greenberg, J. Am. Chem. Soc., 2012, 134, 12478–12481 CrossRef CAS PubMed.
- S. A. Herbert and G. E. Arnott, Org. Lett., 2010, 12, 4600–4603 CrossRef CAS PubMed.
- Selected examples for the transition-metal-catalyzed direct coupling reaction of aromatic carbon with chalcogen, see
(a) L. D. Tran, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237–18240 CrossRef CAS PubMed;
(b) M. Xu, X.-H. Zhang and P. Zhong, Synth. Commun., 2012, 42, 3472–3481 CrossRef CAS;
(c) M.-L. Zhang, S.-H. Zhang, C.-D. Pan and F. Chen, Synth. Commun., 2012, 42, 2844–2853 CrossRef CAS;
(d) C.-M. Yu, C.-L. Zhang and X.-J. Shi, Eur. J. Org. Chem., 2012, 1953–1959 CrossRef CAS;
(e) Y. Li, K. A. Wheelerb and R. Dembinski, Org. Biomol. Chem., 2012, 10, 2395–2408 RSC;
(f) J.-M. Becht and C. Le Drian, J. Org. Chem., 2011, 76, 6327–6330 CrossRef CAS PubMed;
(g) S.-H. Zhang, P.-C. Qian, M.-L. Zhang, M.-L. Hu and J. Cheng, J. Org. Chem., 2010, 75, 6732–6735 CrossRef CAS PubMed;
(h) M. Martinek, M. Korf and J. Srogl, Chem. Commun., 2010, 46, 4387–4389 RSC;
(i) H.-F. Wang, L.-L. Jiang, T. Chen and Y.-M. Li, Eur. J. Org. Chem., 2010, 2324–2329 CrossRef CAS;
(j) J. Clayden, J. Senior and M. Helliwell, Angew. Chem., Int. Ed., 2009, 48, 6270–6273 CrossRef CAS PubMed;
(k) Z.-Y. Duan, S. Ranjit, P.-F. Zhang and X.-G. Liu, Chem. – Eur. J., 2009, 15, 3666–3669 CrossRef CAS PubMed;
(l) M. Arisawa, T. Suzuki, T. Ishikawa and M. Yamaguchi, J. Am. Chem. Soc., 2008, 130, 12214–12215 CrossRef CAS PubMed.
-
(a) J. Liu, P.-H. Li, W. Chen and L. Wang, Chem. Commun., 2012, 48, 10052–10054 RSC;
(b) L. Bagnoli, C. Scarponi, M. G. Rossi, L. Testaferri and M. Tiecco, Chem.–Eur. J., 2011, 17, 993–999 CrossRef CAS PubMed;
(c) A. L. Stein, D. Alves, J. T. da Rocha, C. W. Nogueira and G. Zeni, Org. Lett., 2008, 10, 4983–4986 CrossRef CAS PubMed;
(d) L. Brandsma, H. D. Verkruijsse, C. Schade and P. von Ragué Schleyer, J. Chem. Soc., Chem. Commun., 1986, 260–261 RSC;
(e) G. A. Russell and J. Hershberger, J. Am. Chem. Soc., 1980, 102, 7603–7604 CrossRef CAS.
-
(a) X.-B. Chen, X.-M. Liu, R. Huang, S.-J. Yan and J. Lin, Eur. J. Org. Chem., 2013, 4607–4613 CrossRef CAS;
(b) F.-C. Yu, R. Huang, H.-C. Ni, J. Fan, S.-J. Yan and J. Lin, Green Chem., 2013, 15, 453–462 RSC;
(c) L.-J. Yang, S.-J. Yan, W. Chen and J. Lin, Synthesis, 2010, 20, 3536–3544 Search PubMed.
-
(a) L.-R. Wen, Q.-C. Sun, H.-L. Zhang and M. Li, Org. Biomol. Chem., 2013, 11, 781–786 RSC;
(b) F.-C. Yu, S.-J. Yan, L. Hu, Y.-C. Wang and J. Lin, Org. Lett., 2011, 13, 4782–4785 CrossRef CAS PubMed;
(c) C. Huang, S.-J. Yan, X.-H. Zeng, X.-Y. Dai, Y. Zhang, C. Qing and J. Lin, Eur. J. Med. Chem., 2011, 46, 1172–1180 CrossRef CAS PubMed;
(d) S.-J. Yan, C. Huang, C.-X. Su, Y.-F. Ni and J. Lin, J. Comb. Chem., 2010, 12, 91–94 CrossRef CAS PubMed.
-
(a) Y.-C. Zhang, Z.-C. Liu, R. Yang, J.-H. Zhang, S.-J. Yan and J. Lin, Org. Biomol. Chem., 2013, 11, 7276–7288 RSC;
(b) M. Li, P. Shao, S.-W. Wang, W. Kong and L.-R. Wen, J. Org. Chem., 2012, 77, 8956–8967 CrossRef CAS PubMed;
(c) L.-R. Wen, C.-Y. Jiang, M. Li and L.-J. Wang, Tetrahedron, 2011, 67, 293–302 CrossRef CAS PubMed.
-
(a) M. Li, Z.-M. Zhou, L.-R. Wen and Z.-X. Qiu, J. Org. Chem., 2011, 76, 3054–3063 CrossRef CAS PubMed;
(b) L.-R. Wen, C. Liu, M. Li and L.-J. Wang, J. Org. Chem., 2010, 75, 7605–7614 CrossRef CAS PubMed.
-
(a) J.-W. Gu, W.-T. Xiong, Z.-H. Zhang and S.-Z. Zhu, Synthesis, 2011, 21, 1717–1722 Search PubMed;
(b) S.-J. Yan, Y.-J. Liu, Y.-L. Chen, L. Liu and J. Lin, Bioorg. Med. Chem. Lett., 2010, 20, 5225–5228 CrossRef CAS PubMed.
-
(a) M. Yaqub, R. Perveen, Z. Shafiq, H. Pervez and M. N. Tahir, Synlett, 2012, 23, 1755–1758 CrossRef CAS PubMed;
(b) S.-J. Yan, Y.-L. Chen, L. Liu, Y.-J. Tang and J. Lin, Tetrahedron Lett., 2011, 52, 465–467 CrossRef CAS PubMed.
-
(a) L.-R. Wen, Z.-R. Li, M. Li and H. Cao, Green Chem., 2012, 14, 707–716 RSC;
(b) F.-C. Yu, S.-J. Yan, R. Huang, Y.-J. Tang and J. Lin, RSC Adv., 2011, 1, 596–601 RSC;
(c) S.-J. Yan, Y.-L. Chen, L. Liu, N.-Q. He and J. Lin, Green Chem., 2010, 12, 2043–2052 RSC;
(d) S.-J. Yan and J. Lin, Chin. J. Org. Chem., 2010, 30, 465–468 CAS.
- A. Alizadeh, J. Mokhtari and M. Ahmadi, Tetrahedron, 2012, 68, 319–322 CrossRef CAS PubMed.
- I. Koca, S. H. Ungoren, I. E. Kibriz and F. Yilmaz, Dyes Pigm., 2012, 95, 421–426 CrossRef CAS PubMed.
- Reviews on heterocyclic ketene aminals, see:
(a) M.-X. Wang and Z.-T. Huang, Prog. Nat. Sci., 2002, 12, 249–257 CAS;
(b) M.-X. Wang and Z.-T. Huang, Prog. Nat. Sci., 1999, 9, 971–983 Search PubMed;
(c) Z.-T. Huang and M.-X. Wang, Heterocycles, 1994, 37, 1233–1262 CrossRef CAS;
(d) K.-M. Wang, S.-J. Yan and J. Lin, Eur. J. Org. Chem., 2014, 1129–1145 CrossRef CAS.
-
(a) Z.-T. Huang and Z.-R. Liu, Chem. Ber., 1989, 122, 95 CrossRef CAS;
(b) M.-X. Wang and Z.-T. Huang, J. Org. Chem., 1995, 60, 2807–2811 CrossRef CAS;
(c) X.-P. Nie, M.-X. Wang and Z.-T. Huang, Synthesis, 2000, 10, 1439–1443 CrossRef PubMed;
(d) F.-C. Yu, Z.-Q. Chen, X.-P. Hao, X.-Y. Jiang, S.-J. Yan, J. Lin, RSC Advances, 2013, 3, 13183–13192 Search PubMed.
-
(a) W. D. Kollmeyer, US. Pat., 4053622, 1977, Chem. Abstr., 1978, 88, 37797h;
(b) Z.-T. Huang, J.-C. Wang and L.-B. Wang, Synth. Commun., 1996, 26, 2285–2295 CrossRef CAS.
-
(a) C.-Y. Yu, S.-J. Yan, T. Zhang and Z.-T. Huang, CN Pat., 101041660B, 2006;
(b) C.-Y. Yu, S.-J. Yan, Z.-T. Huang and A.-J. Yu, CN Pat., 100537578C, 2006.
- B. Liu, M.-X. Wang and Z.-T. Huang, Synth. Commun., 1999, 29, 4241–4250 CrossRef CAS.
- K. Baum, N. V. Nguyen, R. Gilardi, J. L. Flippen-Anderson and C. George, J. Org. Chem., 1992, 57, 3026–3030 CrossRef CAS.
- F. A. Davis, W. A. R. Slegeir, S. Evans, A. Schwartz, D. L. Goff and R. Palmer, J. Org. Chem., 1977, 42, 967–972 CrossRef CAS.
-
(a) Z.-T. Huang and M.-X. Wang, Synthesis, 1992, 12, 1273–1276 CrossRef PubMed;
(b) Z. J. Li and D. Charles, Synth. Commun., 2001, 31, 527–533 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 949284 (3g). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra02519a |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of HKAs is highly polarized.19 This leads to higher electron density of the α-carbon (C3) than that of the secondary amino groups (N1 and N5). Consequently, the substituted targets of the α-carbon have been obtained with high selectivity via alkylation,20 acylation,21 glycosation,22 halogenations,23 and nitration reactions.24 However, to the best of our knowledge, no information is available on the formation of C–chalcogen bonds via the coupling reaction of HKAs with chalcogen. Herein, we report the first example of an AgOAc-mediated direct coupling reaction of HKAs 1 with diaryl dichalcogenides 2 that leads to the regioselective functionalization of the α-carbon with excellent yields (83–98%) by a facile post-treatment protocol. The scope of the reaction encompasses HKAs and diaryl dichalcogenides bearing different sized rings, substitution patterns, and functional groups, which suggests that these novel synthons will be suitable for further synthesis of diverse heterocyclic architectures.
C) of HKAs is highly polarized.19 This leads to higher electron density of the α-carbon (C3) than that of the secondary amino groups (N1 and N5). Consequently, the substituted targets of the α-carbon have been obtained with high selectivity via alkylation,20 acylation,21 glycosation,22 halogenations,23 and nitration reactions.24 However, to the best of our knowledge, no information is available on the formation of C–chalcogen bonds via the coupling reaction of HKAs with chalcogen. Herein, we report the first example of an AgOAc-mediated direct coupling reaction of HKAs 1 with diaryl dichalcogenides 2 that leads to the regioselective functionalization of the α-carbon with excellent yields (83–98%) by a facile post-treatment protocol. The scope of the reaction encompasses HKAs and diaryl dichalcogenides bearing different sized rings, substitution patterns, and functional groups, which suggests that these novel synthons will be suitable for further synthesis of diverse heterocyclic architectures.





