Physicochemical characterization of diacyltetrol-based lipids consisting of both diacylglycerol and phospholipid headgroups†
Narsimha Mamidi‡
a,
Sukhamoy Gorai‡a,
Bolledu Ravib and
Debasis Manna*a
aDepartment of Chemistry, Indian Institute of Technology Guwahati, Assam 781039, India. E-mail: dmanna@iitg.ernet.in; Fax: +91 361 258 2349; Tel: + 91 361 258 2325
bDepartment of Chemical Engineering, Indian Institute of Technology Guwahati, Assam 781039, India
First published on 8th May 2014
Abstract
We describe the synthesis of diacyltetrol-based hybrid lipids in which one of the hydroxymethyl groups is modified with an anionic phospholipid headgroup. The hybrid lipids form a monolayer at the air–water interface. In aqueous solution, these lipids form stable liposomes that exhibit a negative surface potential across a wide pH range. The liposomes aggregate in the presence of Ca2+ ions and release encapsulated cationic reporter rhodamine 6G (R6G) at a faster rate than anionic reporter carboxyfluorescein (CF). The hybrid lipids strongly interact with the C1b subdomain of the protein kinase C (PKC)-θ isoform. These new lipids structurally mimic diacylglycerol and conventional phospholipids, and provide an opportunity to explore their physicochemical properties.
Introduction
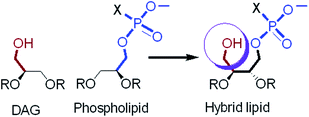
The lipophilic second messenger sn-1,2-diacylglycerols (DAGs) have been long known to be essential intermediates in de novo lipid biosynthesis. The DAGs produced at internal membranes also induce membrane targeting and activation of DAG receptor or effector proteins.1–5 Mammalian cells have low concentrations of DAGs under equilibrium conditions. However, stimulation of phosphatidylinositol-specific phospholipase C (PI-PLC) enzyme by a wide range of G-protein-coupled receptors and receptor tyrosine kinases catalyze the hydrolysis of phosphatidylinositol-4,5 bisphosphate [PtdIns(4,5)P2] to generate DAGs and inositol-1,4,5-triphosphate [(1,4,5)IP3]. The DAGs are also produced from phosphatidylcholine (PC) by concerted action of phospholipase D (PLD) and phosphatidic acid phosphohydrolase, seemingly at the ER and Golgi membranes. DAGs play a vital role in a variety of cellular processes, including cell proliferation, malignant transformation, apoptosis, and others.6,7 Serine–threonine kinase family of proteins are the major cellular receptors of DAGs and has been implicated in many types of cancer.8–10 Anionic phospholipids such as phosphatidic acid (PA), phosphatidylserine (PS), and phosphatidylglycerol (PG) are crucial lipids in the membranes and have been identified as key bioactive molecule in a variety of signalling pathways, and also involved in lipid biosynthesis.11,12Experimental evidences suggest that conventional anionic lipids like PA, PS, and PG enhance the DAG dependent membrane binding affinity and activity of those proteins. These anionic lipids interact with the effector proteins through the positively charged binding surface.13–16 It is also documented that these anionic lipids are specific activators of several DAG receptor proteins. Phosphatidylserine is a specific activator of protein kinase C (PKC) isoenzymes.17 The reported DAG binding mechanism shows that the effector proteins first non-specifically interact with the membrane anionic phospholipids and then specifically bind with DAGs.18 However, the reported experimental measurements used either only DAG or a combination of separate DAG and anionic lipid molecules in solution or under liposomal environment to determine the DAG dependent membrane binding capabilities of the effector proteins.14,15,19 Therefore, it is difficult to understand the role of both the lipid headgroups simultaneously in their effector protein binding capabilities. In addition, the modification of natural DAG structure, to attain higher membrane localization and activation of the targeted proteins in the presence of anionic lipids have been reported on several occasions,20–22 surprisingly, the design and synthesis of lipids having both DAG and anionic lipid headgroups within the same moiety (Fig. 1) have never been attempted. In this report, we investigate how the presence of both DAG and conventional anionic lipid headgroups within the diacyltetrol (DAT) moiety alters the physicochemical properties of the monolayer and bilayer. These properties are the basis for details biochemical and other studies. These hybrid lipids differ structurally from natural lipids in two ways-the lipid backbone and the headgroup. These hybrid lipids have tetrol backbone; unlike the natural lipids and the polar region of each lipid contain a DAG and anionic phospholipid headgroup.
Changes of DAT lipid headgroup has been explored for the development of protein activators and biologically active lipid probes;23–25 however, to the best of knowledge coupling of DAG headgroup with PA/PS/PG headgroup through a diacyltetrol moiety have not been reported so far. The PA, PS, and PG headgroups are well hydrated, maintain their anionic character over a wide pH range, and interact strongly with divalent cations, all properties that make it ideal for its primary role in cell membranes.26–28 Because of the biological importance of these naturally occurring lipids, synthesized hybrid lipids could exhibit similar or better activities, allowing them to adopt a therapeutic role as well as serving as a structural component in a liposome bilayer for studying membrane biophysics.
We are interested in learning the consequence on monolayer and bilayer properties of the DAT-based hybrid lipids that contain both DAG and phospholipid headgroups. We hypothesize that the addition of hydroxyl group to the phospholipids could strongly increase their ability to form hydrogen bonds, which can influence the self-association behaviour and the ability to interact with the guest molecule. In this text, we report the synthesis of DAT-based hybrid lipids and characterization of physicochemical properties including, air–water interfacial behaviour, surface potential change, interaction with divalent cation, permeability of anionic and cationic reporters, and demonstration of their ability to activate selective cytosolic proteins.
Experimental section
Materials and methods
All reagents were purchased from Sigma (St. Louis, MO) and Merck (Mumbai, India) and used directly without further purification. Dry solvents were obtained according to the reported procedures. Column chromatography was performed using 60–120 mesh silica gel. Reactions were monitored by thin-layer chromatography (TLC) on silica gel 60 F254 (0.25 mm). 1H NMR, 13C NMR, and 31P NMR spectra were recorded at 400, 100, and 162 MHz, respectively using a Varian AS400 spectrometer. Coupling constants (J values) are reported in Hertz, and chemical shifts are reported in parts per million (ppm) downfield from tetramethylsilane using residual chloroform (δ = 7.24 for 1H NMR, δ = 77.23 for 13C NMR) as an internal standard. 31P NMR spectra were recorded in CDCl3 and calibrated to external standard 85% H3PO4. Multiplicities are reported as follows: s (singlet), d (doublet), t (triplet), m (multiplet), and br (broadened). Melting points were determined using a melting point apparatus and are uncorrected. Mass spectra were recorded using a Waters Q-TOF Premier mass spectrometry system, and data were analyzed using the built-in software. 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine (DPPS), 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA) and 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DPPG) were purchased from Avanti Polar Lipids (Alabaster, AL). Ultrapure water (Milli-Q system, Millipore, Billerica, MA) was used for the preparation of buffers.Interfacial behavior measurement by Langmuir trough techniques
Stock solutions of the lipids were prepared in a chloroform–methanol mixture [4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)]. The interfacial behaviors of these lipids were investigated by monitoring their arrangement at the air–water interface using Langmuir trough technique (NIMA technology Ltd) according to the Wilhelmy plate method.29 The sub-phase consisted of Milli-Q water (Millipore, Bedford, MA) and had a resistance of 18.4 MΩ cm. Before each experiment, the cleanliness of the sub-phase was verified by repeated compression without lipids. If the surface pressure changed by less than 0.2 mN m−1 the surface was considered to be clean. Lipid solutions (25 μL of 0.5 mg mL−1 stock solution) were deposited onto the surface of the sub-phase. After the solvent had been allowed to evaporate for 10 min, the monolayer was compressed at a constant rate of 10 mm min−1, and the surface pressure (π)–area (A) isotherm was continuously recorded. All experiments were conducted at room temperature. The π–A isotherm was analyzed by inbuilt software. Extrapolation of the part of the isotherm with the highest slope to zero surface pressure gave the limiting area per molecule A0 in square angstroms.
1 (v/v)]. The interfacial behaviors of these lipids were investigated by monitoring their arrangement at the air–water interface using Langmuir trough technique (NIMA technology Ltd) according to the Wilhelmy plate method.29 The sub-phase consisted of Milli-Q water (Millipore, Bedford, MA) and had a resistance of 18.4 MΩ cm. Before each experiment, the cleanliness of the sub-phase was verified by repeated compression without lipids. If the surface pressure changed by less than 0.2 mN m−1 the surface was considered to be clean. Lipid solutions (25 μL of 0.5 mg mL−1 stock solution) were deposited onto the surface of the sub-phase. After the solvent had been allowed to evaporate for 10 min, the monolayer was compressed at a constant rate of 10 mm min−1, and the surface pressure (π)–area (A) isotherm was continuously recorded. All experiments were conducted at room temperature. The π–A isotherm was analyzed by inbuilt software. Extrapolation of the part of the isotherm with the highest slope to zero surface pressure gave the limiting area per molecule A0 in square angstroms.
Measurement of transition temperature
Liposome of only lipids were prepared by thin film hydration in 10 mM Tris buffer, pH 7.4, containing 150 mM NaCl, to a final concentration of 15 mM lipid, followed by brief sonication at 50 °C for 2 minutes.30 Then 200 μL of each liposomal solution were added into each calorimeter chamber with buffer used in the specific liposome preparation as the standard. The measurements were carried out by using a Q20-DSC (TA Instruments) calorimeter. Data was collected over a range of 20 to 90 °C at 1 degree per minute.Transmission electron microscopy
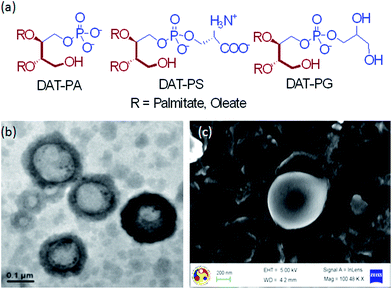
Lipid formulations (lipid16/18–cholesterol (6/4; mol mol−1)) were prepared by thin film hydration method (no extrusion method was used) in 10 mM Tris buffer, pH 7.4 (final concentration 2 mM). However, the identity of the C–C double bond of DAT-PX18 was not altered after hydration, as measured by 1H NMR spectroscopy. Negative staining technique were used to prepare the samples before the transmission electron microscope (TEM) imaging.29,30 Briefly, 10 μL of a liposomal solution was placed onto a carbon-coated copper grid and allowed to absorb for 1 minute. The grid was carefully blotted with filter paper. Then a 1% solution of uranyl acetate in water was dropped on the grid and allowed to sit for 1 minute. The uranyl acetate solution was then removed and the grid was washed once with water and excess water was removed, and the grid was allowed to dry. A JEOL JEM 2100 transmission electron microscope (operated at a maximum accelerating voltage of 200 kV) was used to capture images of liposomes formed on the carbon-coated copper grid.pH dependent zeta potential measurements
Liposomes of the selected lipids (lipid16/18) were prepared according to the mentioned procedure in 5 mM Tris buffer, pH 8.6, containing 5 mM NaCl. The unilamellar vesicles were prepared by extruding through a polycarbonate membrane (100 nm) using a handheld mini-extruder (Avanti Polar Lipids, Alabaster, AL) at room temperature.30 Zeta potential measurements of liposomes at different pH values were performed using a Zetasizer Nano ZS90 (Malvern, Westborough, MA). Isosmotic buffers containing 10 mM buffering agent and 10 mM salt at different pH values were freshly prepared and used for the experiments. The buffering agents at different pH were selected based on their pKa values. Citric acid and trisodium citrate was used for pH = 3.0–6.5; 3-(N-morpholino)propanesulfonic acid (MOPS) was used for pH 7.0, tris(hydroxymethyl)aminomethane (Tris)–HCl was used for pH = 7.5–8.5. Liposomal solution was diluted into 1 mL of isosmotic buffer (final lipid concentration was 133 nM) in a disposable capillary cell (DTS1061) for zeta potential measurements. All the measurements were performed three times per sample and averaged to give the final value.Calcium-induced zeta potential measurements
Liposome preparation used in the pH dependent zeta potential measurements were also used for the calcium-induced zeta potential measurements in 20 mM of 2-(N-morpholino)ethanesulfonic acid (MES) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, pH 7.4. Liposomal solution was added into 1 mL of 10 mM HEPES buffer, pH 7.4 (final lipid concentration was 67 nM) with various amounts of CaCl2 and NaCl.30 NaCl was added along with CaCl2 to maintain a constant ionic strength across all Ca2+ ion concentrations. Ionic strengths of the solutions were calculated according to the Debye–Hückel model, where the ionic strength (I) = 0.5(4[Ca2+] + [Cl−] + [Na+]). Zeta potential measurements of liposomes were performed using a Zetasizer Nano ZS90.Vesicle leakage study
Lipid formulations (lipid16/18–cholesterol (6/4)) were dried out from chloroform solutions to form a thin film. The films were hydrated with 10 mM Tris buffer, pH 7.4, containing 2 mM carboxyfluorescein (CF) or 100 μM rhodamine 6G (R6G). The preparations were then sonicated at 50 °C for 2 minutes and free CF or R6G was removed by size exclusion chromatography on a PD-10 Sephadex column (Sigma, St. Louis, MO) by eluting with 10 mM HEPES buffer, pH 7.4, containing 92 mM NaCl and 8 mM NaN3. Then, each purified liposome solution was added into 1 mL of the elution buffer (final lipid concentration was 1.7 mM). The release of CF and R6G was measured by monitoring the emission signals at 516 nm (λex = 485 nm) and 554 nm (λex = 530 nm), respectively.30 All the fluorescence measurements were performed using Fluoromax-4 spectrofluorometer (Horiba Scientific, Singapore) at room temperature. Total fluorescence of CF/R6G was measured by liposome lysis using TRITON X-100 surfactant (final concentration was 2 mM). Percent release was calculated using the following relation: percentage release at time, (t) = (measured fluorescence at time, (t))/(total fluorescence from lysed liposomes) × 100.Surface plasmon resonance analysis
All surface plasmon resonance (SPR) measurements were performed (at 25 °C, flow rate of 30 μL min−1) using a lipid-coated L1 sensorchip in the Biacore X100 (GE Healthcare) system as described earlier.31 The vesicles composed of PC/PE/PS (60/20/20) and PC/PE/DAT-PX16 (75/20/5) was used as control and active surface, respectively. The DAT-PX16 stands for PA16, PS16 and PG16 containing hybrid lipids.Results and discussions
Orientation of hybrid lipids at interface
Lipid monolayers are considered as a model system for biological membranes. Monolayer studies provide an insight into how chemical structure, and physical behavior of lipids, including intermolecular and intramolecular interactions modify the assembly of the molecules at the air–water interface.29,32 To obtain information about the interfacial behaviour of the pure hybrid lipids only we investigated their arrangement at the air–water interface using Langmuir trough technique. Presence of other lipids or cholesterol could affect the monolayer properties of hybrid lipids. The surface pressure (π)–area per molecule (A) isotherms and elastic compressibility moduli (Cs−1) as a function of molecular area of the saturated hybrid lipids, displayed a bit similar pattern as of the conventional phospholipids (PA/PS/PG) monolayers, with transition from gaseous phase (G) to liquid-expanded (LE) state (Fig. 3b). The isotherms also indicate that these lipids become partially soluble in the water subphase with the increase in lateral pressure. This phenomenon is supported by their lower mean molecular area values (<35 Å2), and additional ordering in the LE phase.33 It is also reported that DAGs with unsaturated ‘tail’ generated by PI-PLC are abundant in cell membranes and more potent in PKC activation than the saturated forms produced by other pathways. For this reason, we have also used unsaturated hybrid lipids to investigate their interfacial behavior. The characteristic data also suggests that, the unsaturated hybrid lipids form closely packed stable monolayer with sequential phase transition from G to LE phase and liquid expanded–liquid condensed coexistence (LE–LC) region, and a more condensed phase during the compression process. Fig. 4 represents a comparison in the phase transition pattern of the saturated and unsaturated hybrid lipids under the similar experimental conditions. The π–A isotherm and calculated compressibility modulus (Cs−1) clearly showed their difference in phase transition behavior. The presence of LE–LC is indicated by the plateau region in the isotherm. However, after LE–LC phase the surface pressure increases abruptly with compression (particularly for DAT-PS18 and DAT-PG18), indicating a more densely packing state of the lipids (Fig. S36†). The additional ordering in π–A isotherm and higher collapsed area values of DAT-PX18 than DAT-PX16 lipids could be due to the presence of unsaturated ‘tail’ (oleic acid) that led to the formation of expanded monolayer compared to the saturated ones (Table S2†). The cis-double bond in the oleic acid prevents them from packing together tightly, than the saturated fatty acid or even fatty acid with trans-double bond. A similar increase was also reported for unsaturated fatty acids over saturated fatty acids only.34 However, the change in higher collapsed areas for hybrid lipids over only fatty acid could be due to the presence of additional two lipid headgroups and two unsaturated ‘tail’ within the same moiety. Interestingly, the DAT-PS and DAT-PG lipids showed higher A0 values than DAT-PA, which could be related to the steric hindrance caused by their headgroups. Presence of additional DAG headgroup in these hybrid lipids appeared to have an observable effect on the overall conformational flexibility of the hydrophilic group, result in an unrelated arrangement of the hybrid lipids (in particular for DAT-PX18) than conventional phospholipids. Significantly higher collapse pressure, than the biological membrane also supported the tighter monolayer formation by the hybrid lipids.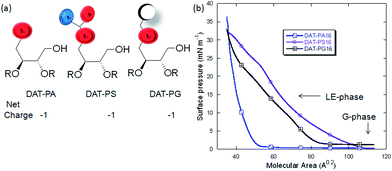 | ||
| Fig. 3 Illustration of charge orientation and net charge of the hybrid lipids (a). Surface pressure (π)–molecular area (A) isotherm of the saturated hybrid lipids (b). | ||
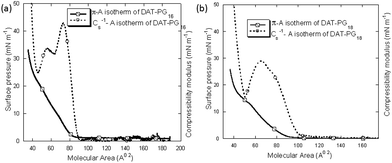 | ||
| Fig. 4 Surface pressure (π)–molecular area (A) isotherm (−) and compressibility modulus (Cs−1)–A (▪▪▪) isotherms of DAT-PG16 (a) and DAT-PG18 (b). | ||
Thermotropic phase behavior of bilayers composed of synthetic lipids
To understand the role of additional DAG headgroup in the thermotropic phase behavior of the bilayer of pure hybrid lipids, we measured transition temperature (Tm) by differential scanning calorimetry (DSC). We first collected the TEM and FE-SEM images of all the lipids to understand their vesicle forming capabilities (Fig. 2b and c, S37 and S38†). The images clearly showed that all lipids form stable liposomes under the experimental conditions. The transition of lipids from an gel state to a more fluid state is often dictated by the chain length, degree of unsaturation of the hydrophobic tails and the nature of headgroups .29,35 The Tm values were found to be 67.6 °C, 57.5 °C, and 44.5 °C for DAT-PA16, DAT-PS16, and DAT-PG16, respectively. The values are slightly higher than the Tm values of DPPA (63.9 °C), DPPS (55.8 °C), and DPPG (43.8 °C), indicating that DAG headgroups influence Tm values to some extent (Fig. S39†). Interestingly, lipids with PS and PA headgroups have substantially elevated Tm values relative to lipids with PG headgroups. The PS and PG headgroups are extending away from the bilayer interface in comparison with the lipids with PA headgroups. Therefore, the higher Tm values of DAT-PS and DAT-PA in comparison with DAT-PG could be due to hydration of the lipid headgroups. The anionic lipid headgroups have larger hydration capabilities, but the degree of hydration depends on the effective size of the polar headgroup and its geometry. Hydration loosens the packing of the lipids due to the weakening of electrostatic interaction and/or accommodation of water molecules between adjacent polar headgroups, resulting in lower Tm values. Thus, DSC results also indicate that DAT-PG have larger hydration capabilities than DAT-PS and DAT-PA.Surface potential of the synthetic lipid containing liposomes
We investigated the influence of DAG headgroup in the hybrid lipids on the overall surface potential of their liposomes. Ionization behavior of hybrid lipid-associated liposomes is essential for the development of structure–activity relationship, which can be used in understanding their protein interaction properties. The pH-dependent surface potential of the liposomes were compared to zeta potential value, a surrogate measure of surface potential.36,37 The conventional anionic lipids showed highly negative surface potential at high pH and remained negative and constant as pH decreases. The liposomes of hybrid lipids also showed similar ionization behavior; however, the magnitudes of surface potential were slightly higher than that of conventional anionic lipids (Fig. 5a).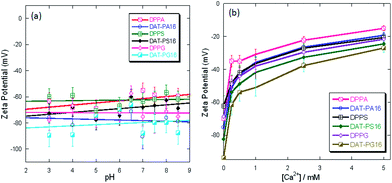 | ||
| Fig. 5 Zeta potential of the liposomes at different solution pH (a). Shift in zeta potential in the presence of Ca2+ ion (b). | ||
Therefore, the hybrid lipids form liposomes with negative surface potential and the presence of additional DAG headgroup reduce the phosphate pKa value to some extent. The surface potential measurements also reveal subtle differences between the hybrid lipids with saturated and unsaturated hydrophobic tails (Fig. 5a and Fig. S40†).
Divalent cations are often present in biological systems and can interact with the lipid headgroups to induce aggregation, fusion, and modify the surface potential. This liposomal aggregation occurs when divalent cations such as Ca2+ forms bridges between the outer membranes of two adjacent liposomes.30,38,39 The liposomal aggregation also depends on the rate of liposomal collisions in the solution, Ca2+ concentration, and the strength of interaction between Ca2+ and the phosphate group of the lipids.40 To determine if the presence of Ca2+ altered the electronic properties of the liposomes of hybrid lipids, the surface potentials were measured in the presence of increasing concentration of Ca2+. The surface potential for saturated hybrid lipids increased with the increasing Ca2+ concentration; however, the surface potential values remained negative up to 5 mM Ca2+ concentration (Fig. 5b). The overall magnitudes of the increase in surface potential values were slightly higher for liposomes of hybrid lipids in comparison with the conventional phospholipids. This indicates that Ca2+ strongly interacts with the surface of the liposomes of hybrid lipids and conventional phospholipids due to the location of the phosphate group. We hypothesize that the presence of hydroxymethyl group in the hybrid lipids may be involved in additional interaction with the Ca2+ allowing the liposomes to either aggregate or fuse more. Therefore, not only the surface charge but also their localization at the bilayer interface and adjacent groups also determine the strength of interaction between liposomes and ions/charged molecules.
This measurement pointed out that liposomal aggregation, fusion, and/or modification of the surface charge are primarily associated with the lipid headgroups. Therefore, we presume that the unsaturated hybrid lipids would show a similar change in surface potential behaviour in the presence of increasing Ca2+ concentration.
Leakage of hybrid lipid containing liposomes
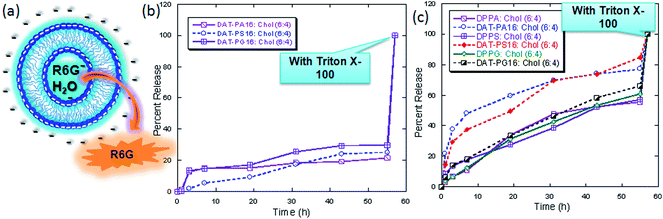
The anionic headgroups of the lipids are known to attract positively charged molecules or ions towards the surface of the liposomes and sometimes into the hydrophobic core of the bilayer, due to the electrostatic interaction. This results in an increase permeability of the positively charged molecules, relative to the negatively charged molecules.41,42 To follow this liposomal phenomenon, we measured the permeability of water-soluble anionic reporter carboxyfluorescein (CF) and cationic reporter rhodamine 6G (R6G) through the liposomes of hybrid lipids and conventional anionic lipids, containing 40 mol% cholesterol at 37 °C (Fig. 6). During this measurement, we used only saturated hybrid lipids because the unsaturated hybrid lipids also show a similar leakage profile (data not shown). We then investigate the R6G release profiles of the pure hybrid lipids; however, the release rate was too fast to study other physicochemical properties of the vesicle leakage phenomenon (Fig. S41†). Therefore, we used cholesterol containing liposomes for further studies. The presence of cholesterol in the liposome decreases the membrane fluidity and induces order in the gel state by increasing packing of ‘tail’ of the lipids. Cholesterol also decreases the lateral diffusion and therefore lowers the effect of membrane transport. Biological membranes also contain as high as 20–40 mol% cholesterol. Presence of such high cholesterol amount also reduce the permeability of Na+, ethanol and other small solute molecules, which is considered to be one of the important characteristics for the sustainability of the liposomes and their usefulness in other biological applications.43–45 The release rate clearly showed that R6G have better permeability than CF, through the liposomes containing hybrid lipids. However, the permeability of R6G was much faster through the liposomes of hybrid lipids than conventional anionic lipids. This could be due their additional interaction (possibly through hydrogen bond formation with the DAG head group) with the positively charged molecules, which was already observed during the Ca2+-induced surface potential measurements. In addition, the R6G release profiles of the hybrid lipid containing liposomes in the presence of different concentration of Ca2+ also support this phenomenon (Fig. 7). The results showed that the presence of Ca2+ strongly influence the vesicle leakage profile of the hybrid lipids and cholesterol containing liposomes (Fig. S42†). This is another important characteristic of these lipids for their plausible use as building blocks for liposomes in drug delivery since a significant amount of Ca2+ and other divalent cations are present in the biological media.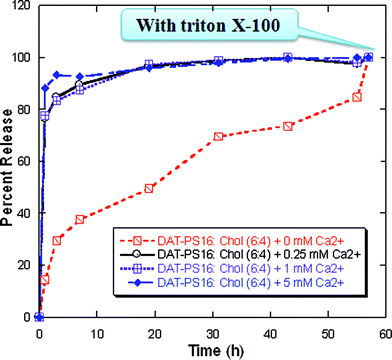 | ||
| Fig. 7 Rhodamine 6G (R6G) release profile from cholesterol containing DAT-PS16 liposomes in the absence and presence of different Ca2+ concentration. | ||
These results suggest that the presence of additional hydroxymethyl group in the hybrid lipids plays a significant role in releasing positively charged molecule. It is also important to note that, the DAT-PA16, DAT-PS16 liposomes are preferentially permeable to positively charged molecules than that of DAT-PG16, supporting that extent of localization, and exposure of the polar headgroup in the aqueous phase plays a crucial role in releasing positively charged molecules. Whereas, mixed lipids containing liposomes could be used to prepare liposomes with adjustable surface charge to generate adaptable content release profile for charged compounds (Fig. S43†).
Interaction with protein under liposomal environment
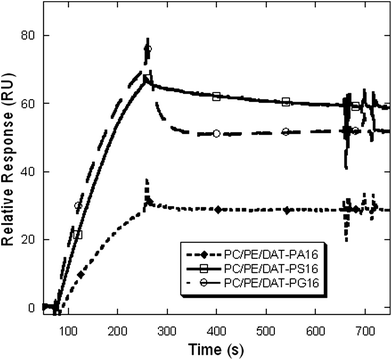
To explore their ability in protein binding and activation, we measured binding parameters of the saturated hybrid lipids with the C1b subdomain of PKC-θ using surface plasmon resonance (SPR) analysis. However, this quantitative in vitro protein binding measurement is very important to determine their affinity and specificity because the cellular membranes are enriched with the anionic phospholipids. The C1 and C2 domains of PKC isozymes have strong binding affinities for anionic phospholipids, so it is difficult to use the whole-enzyme to characterize hybrid lipid binding affinity of PKCs. Therefore isolated C1 domain was chosen for the protein binding affinity measurement studies. The PKCθ-C1b subdomain has sufficiently stronger binding affinity for DAG in the presence of anionic phospholipids (Fig. 8). The measured binding affinities also showed that hybrid lipids strongly interact with the PKCθ-C1b subdomain, under the experimental condition (Table S3†). The binding parameters are in good correlation with the separate DAG and anionic phospholipids. The SPR sensorgram also showed that the PKCθ-C1b subdomain binding to these lipids is governed to a large extent by slow dissociation rate from the membrane surface, characteristic properties of the PKC-C1 domains (Fig. S44†).46,47 For all the SPR measurements vesicles composed of PC/PE/PS (60/20/20) and PC/PE/DAT-PX16 (75/20/5) was used as control and active surface, respectively to investigate the C1 domain binding affinity for the hybrid lipids only.18,23,31 Zwitterionic PC and PE are also known to stabilize the liposomes. It is documented that the unsaturated DAGs have slightly stronger binding affinity for the PKC-C1 domains than the saturated DAGs.3,48 Therefore, we presume that unsaturated hybrid lipids would also show a similar and/or better protein binding affinity under these experimental conditions.Overall, these physicochemical studies of the synthesized hybrid lipids varying in degree of unsaturation and type of polar headgroups demonstrate that the presence of additional hydroxymethyl group does not significantly alter the monolayer and bilayer forming properties than the natural anionic phospholipids. But, the presence of unsaturated ‘tails’ alter the molecular arrangements of the hybrid lipids at the air–water interface. The TEM and FE-SEM images show that all lipids are capable of forming stable liposomes under the experimental conditions. The surface potential of the hybrid lipids containing liposomes is also not significantly affected by the pH of the solution and the presence of Ca2+ in the solution. However, the additional hydroxymethyl group assists the hybrid lipids to strongly interact with the positively charged molecules, which significantly alter their release profile from vesicles. Therefore, the hybrid lipids could serve as a useful tool for studying properties related to drug delivery. The results also show that the hybrid lipids can influence the membrane binding properties of proteins and their binding affinity difference could be primary due to their preference for anionic phospholipid headgroups. This indicates that these hybrid lipids can be further used as enzyme specific activators.
Conclusions
We have synthesized a new class of diacyltetrol based hybrid lipids, in which both the DAG and anionic phospholipid (PA/PS/PG) headgroups are present within the same molecule. The results provide a detail physicochemical characterization of synthesized hybrid lipids varying in fatty acids and polar headgroups. Additional DAG headgroup has no significant effect on the π–A isotherms of the saturated phospholipids, but the presence of unsaturated ‘tail’ strongly affect their monolayer behaviour. The liposomes have negative surface potential across a wide pH range, strongly interact with Ca2+, and release encapsulated cationic reporter at a faster rate. The presence of diacylglycerol headgroup within the hybrid lipids effectively enhance their interaction properties with the positively charge molecules than conventional phospholipids. The PKCθ-C1b subdomain strongly interacts with the hybrid lipids under liposomal environment and their binding affinities are better and/or comparable with the reported diacylglycerol binding affinity in presence of conventional anionic lipids. Therefore, diacyltetrol based hybrid lipids represent a distinctive family of synthesized lipids that structurally mimic the DAG and conventional anionic lipids can be exploited to generate biologically active molecules for an activation of specific cytosolic proteins, and provide an opportunity to explore its biological activities.Acknowledgements
We gratefully acknowledge CSIR (02(0090)/12/EMR-II), DST-FIST (SR/FST/ETII-028/2010) and DBT programme support (no. BT/01/NE/PS/08) Govt. of India for financial support. We are thankful to Center for Nanotechnology and CIF for instrumental support. Special thanks are due to Dr Debapratim Das for his helpful suggestions.Notes and references
- F. Battaini and D. Mochly-Rosen, Pharmacol. Res., 2007, 55, 461 CrossRef CAS PubMed.
- G. Boije af Gennas, V. Talman, O. Aitio, E. Ekokoski, M. Finel, R. K. Tuominen and J. Yli-Kauhaluoma, J. Med. Chem., 2009, 52, 3969 CrossRef CAS PubMed.
- M. J. Wakelam, Biochim. Biophys. Acta, 1998, 1436, 117 CrossRef CAS.
- E. M. Griner and M. G. Kazanietz, Nat. Rev. Cancer, 2007, 7, 281 CrossRef CAS PubMed.
- S. G. Rhee, Annu. Rev. Biochem., 2001, 70, 281 CrossRef CAS PubMed.
- J. M. Boon, T. N. Lambert, A. L. Sisson, A. P. Davis and B. D. Smith, J. Am. Chem. Soc., 2003, 125, 8195 CrossRef CAS PubMed.
- M. Maceyka, S. G. Payne, S. Milstien and S. Spiegel, Biochim. Biophys. Acta, 2002, 1585, 193 CrossRef CAS.
- E. C. Dempsey, A. C. Newton, D. Mochly-Rosen, A. P. Fields, M. E. Reyland, P. A. Insel and R. O. Messing, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2000, 279, L429 CAS.
- H. J. Mackay and C. J. Twelves, Nat. Rev. Cancer, 2007, 7, 554 CrossRef CAS PubMed.
- B. A. Teicher, Clin. Cancer Res., 2006, 12, 5336 CrossRef CAS PubMed.
- G. Benga and R. P. Holmes, Prog. Biophys. Mol. Biol., 1984, 43, 195 CrossRef CAS.
- G. van Meer, D. R. Voelker and G. W. Feigenson, Nat. Rev. Mol. Cell Biol., 2008, 9, 112 CrossRef CAS PubMed.
- S. Sanchez-Bautista, S. Corbalan-Garcia, A. Perez-Lara and J. C. Gomez-Fernandez, Biophys. J., 2009, 96, 3638 CrossRef CAS PubMed.
- A. C. Newton, Chem. Rev., 2001, 101, 2353 CrossRef CAS PubMed.
- D. Manna, N. Bhardwaj, M. S. Vora, R. V. Stahelin, H. Lu and W. H. Cho, J. Biol. Chem., 2008, 283, 26047 CrossRef CAS PubMed.
- M. Mosior, E. S. Golini and R. M. Epand, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 1907 CrossRef CAS.
- A. C. Newton and L. M. Keranen, Biochemistry, 1994, 33, 6651 CrossRef CAS.
- N. Mamidi, R. Borah, N. Sinha, C. Jana and D. Manna, J. Phys. Chem. B, 2012, 116, 10684 CrossRef CAS PubMed.
- M. Medkova and W. Cho, Biochemistry, 1998, 37, 4892 CrossRef CAS PubMed.
- O. Raifman, S. Kolusheva, M. J. Comin, N. Kedei, N. E. Lewin, P. M. Blumberg, V. E. Marquez and R. Jelinek, FEBS J., 2010, 277, 233 CrossRef CAS PubMed.
- J. H. Kang, S. Benzaria, D. M. Sigano, N. E. Lewin, Y. M. Pu, M. L. Peach, P. M. Blumberg and V. E. Marquez, J. Med. Chem., 2006, 49, 3185 CrossRef CAS PubMed.
- Y. Pu, N. A. Perry, D. Yang, N. E. Lewin, N. Kedei, D. C. Braun, S. H. Choi, P. M. Blumberg, S. H. Garfield, J. C. Stone, D. Duan and V. E. Marquez, J. Biol. Chem., 2005, 280, 27329 CrossRef CAS PubMed.
- N. Mamidi, S. Gorai, R. Mukherjee and D. Manna, Mol. BioSyst., 2012, 8, 1275 RSC.
- M. D. Smith, C. G. Sudhahar, D. Gong, R. V. Stahelin and M. D. Best, Mol. BioSyst., 2009, 5, 962 RSC.
- M. D. Smith, D. Gong, C. G. Sudhahar, J. C. Reno, R. V. Stahelin and M. D. Best, Bioconjugate Chem., 2008, 19, 1855 CrossRef CAS PubMed.
- F. C. Tsui, D. M. Ojcius and W. L. Hubbell, Biophys. J., 1986, 49, 459 CrossRef CAS.
- J. X. Cheng, S. Pautot, D. A. Weitz and X. S. Xie, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9826 CrossRef CAS PubMed.
- D. Bach and I. R. Miller, Chem. Phys. Lipids, 2005, 136, 67 CrossRef CAS PubMed.
- M. Hubert, B. J. Compton, C. M. Hayman, D. S. Larsen, G. F. Painter, T. Rades and S. Hook, Mol. Pharm., 2013, 10, 1928 CrossRef CAS PubMed.
- E. K. Perttu, A. G. Kohli and F. C. Szoka, Jr, J. Am. Chem. Soc., 2012, 134, 4485 CrossRef CAS PubMed.
- D. Manna, A. Albanese, W. S. Park and W. Cho, J. Biol. Chem., 2007, 282, 32093 CrossRef CAS PubMed.
- M. A. Bos and T. Nylander, Langmuir, 1996, 12, 2791 CrossRef CAS.
- X. Chen, Z. Huang, W. Hua, H. Castada and H. C. Allen, Langmuir, 2010, 26, 18902 CrossRef CAS PubMed.
- R. T. Florence and W. D. Harkins, J. Chem. Phys., 1938, 6, 856 CrossRef CAS PubMed.
- C. Huang, Z. Q. Wang, H. N. Lin, E. E. Brumbaugh and S. S. Li, Biochim. Biophys. Acta, Biomembr., 1994, 1189, 7 CrossRef CAS.
- C. L. Walsh, J. Nguyen and F. C. Szoka, Chem. Commun., 2012, 48, 5575 RSC.
- U. Kohler, H. H. Mantsch and H. L. Casal, Can. J. Chem., 1988, 66, 983 CrossRef PubMed.
- J. Wilschut, N. Duzgunes, R. Fraley and D. Papahadjopoulos, Biochemistry, 1980, 19, 6011 CrossRef CAS.
- R. Leventis, J. Gagne, N. Fuller, R. P. Rand and J. R. Silvius, Biochemistry, 1986, 25, 6978 CrossRef CAS.
- A. Portis, C. Newton, W. Pangborn and D. Papahadjopoulos, Biochemistry, 1979, 18, 780 CrossRef CAS.
- H. Hauser, D. Oldani and M. C. Phillips, Biochemistry, 1973, 12, 4507 CrossRef CAS.
- I. V. Khavrutskii, A. A. Gorfe, B. Z. Lu and J. A. McCammon, J. Am. Chem. Soc., 2009, 131, 1706 CrossRef CAS PubMed.
- A. J. Desai and L. J. Miller, Front. Endocrinol., 2012, 3, 123 CAS.
- E. M. Bastiaanse, K. M. Hold and A. Van der Laarse, Cardiovasc. Res., 1997, 33, 272 CrossRef CAS.
- J. H. Ipsen, O. G. Mouritsen and M. Bloom, Biophys. J., 1990, 57, 405 CrossRef CAS.
- H. R. Melowic, R. V. Stahelin, N. R. Blatner, W. Tian, K. Hayashi, A. Altman and W. Cho, J. Biol. Chem., 2007, 282, 21467 CrossRef CAS PubMed.
- W. Cho, J. Biol. Chem., 2001, 276, 32407 CrossRef CAS PubMed.
- R. V. Stahelin, J. Lipid Res., 2009, 50, S299 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic protocols, DSC, vesicle leakage, zeta potential and monolayer formation data, TEM and FESEM images. See DOI: 10.1039/c4ra02495h |
| ‡ N.M. and S.G. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |