Electrospun polycaprolactone membranes incorporated with ZnO nanoparticles as skin substitutes with enhanced fibroblast proliferation and wound healing
Abstract
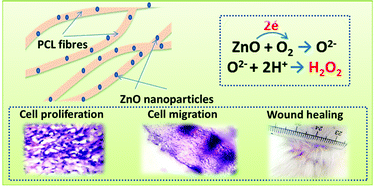
ZnO nanoparticles are well known for their ability to generate Reactive Oxygen Species (ROS) which have a potential role in biological systems. ROS can enhance wound healing by improved cell adhesion and cell migration through growth factor mediated pathways. Here we report the fabrication of electrospun polycaprolactone scaffolds incorporated with ZnO nanoparticles and their ability to perform as skin substitute materials which promote the healing process. The plain or ZnO nanoparticle incorporated PCL membranes were implanted subcutaneously in guinea pigs. Immunological, macroscopical and histological evaluations have shown that the use of membranes containing ZnO nanoparticles enhances the cell adhesion and migration. The ZnO nanoparticle embedded membranes do not show any significant sign of inflammation. The implant also enhanced the wound healing without any scar formation.

 Please wait while we load your content...
Please wait while we load your content...