Triazole-acetate functionalized gold nanoparticles for colorimetric Pb(ii) sensing†
Abstract
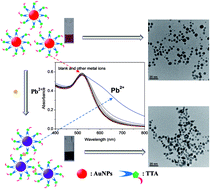
New triazole-acetate functionalized gold nanoparticles (TTA–AuNPs) for sensitive and selective colorimetric detection of Pb2+ were developed. Aggregation of TTA–AuNPs was induced immediately in the presence of Pb2+, yielding a color change from wine-red to purple. This Pb2+-induced aggregation of TTA–AuNPs was monitored by the bare eye and UV-vis spectroscopy with a detection limit of 16.7 nM. TTA–AuNPs showed excellent selectivity toward Pb2+compared to other metal ions through the interaction between the carboxyl group and triazole structure of TTA and Pb2+. The best detection of Pb2+ was achieved in a pH range from 5 to 10. Furthermore, TTA–AuNPs were applied to detect Pb2+ in lake water with low interference.

 Please wait while we load your content...
Please wait while we load your content...