Synthesis of 6-aminophenanthridines via palladium-catalyzed insertion of isocyanides into N-sulfonyl-2-aminobiaryls†
Abstract
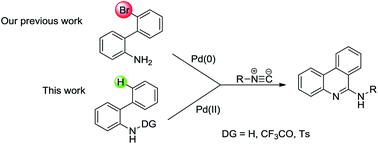
A robust route to a diverse set of 6-aminophenanthridines via palladium-catalyzed C–H activation of N-sulfonyl-2-aminobiaryl and isocyanide insertion is reported. This transformation could also provide an important approach for building core frameworks of conjugated organic polymer materials.

 Please wait while we load your content...
Please wait while we load your content...