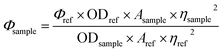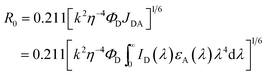A FRET-based ‘off–on’ molecular switch: an effective design strategy for the selective detection of nanomolar Al3+ ions in aqueous media†
Buddhadeb Sena,
Siddhartha Pala,
Somenath Lohara,
Manjira Mukherjeea,
Sushil Kumar Mandalb,
Anisur Rahman Khuda-Bukhshb and
Pabitra Chattopadhyay*a
aDepartment of Chemistry, The University of Burdwan, Golapbag, Burdwan 713104, India. E-mail: pabitracc@yahoo.com; Fax: +91-342-2530452; Tel: +91-342-2558554 ext. 424
bMolecular Biology and Genetics Laboratory, Department of Zoology, Kalyani University, India
First published on 23rd April 2014
Abstract
A new water-soluble rhodamine-based Al3+ ion-selective probe (L1) was synthesised and characterized by physico-chemico and spectroscopic tools. In the presence of a large excess of other competing ions, L1 specifically binds Al3+ ions with a concurrent visually observable change from colorless to pink in electronic spectral behavior, making it possible to detect the presence of Al3+ ions with the naked eye. The addition of Al3+ ions to a solution of L1 in HEPES buffer (1 mM, pH 7.4, 2% EtOH) at 25 °C, results in a decrease in the weak fluorescence intensity at λem = 470 nm, while a new peak (at λem = 588 nm) increases gradually through a fluorescence resonance energy transfer process. This ratiometric enhancement helps to detect Al3+ ions at a very low concentration of 33 nM. The detection limit of L1 for Al3+ ions was estimated to be 6.19 × 10−9 M using the 3σ method. This probe is also useful for imaging Al3+ ions in HeLa cells.
Introduction
Chemosensors for the selective detection of various biologically and environmentally relevant metal ions have attracted great attention because of their potential use in medicine and in environmental research. Aluminum is the third most abundant element on the Earth after oxygen and silicon and there is widespread exposure of humans to aluminum due to its use in food additives, aluminum-based pharmaceuticals and cooking utensils.1,2 After absorption, aluminum is distributed throughout all living tissues in humans and animals and accumulates in bone. Iron-binding proteins (e.g. transferrin C1 and C2, ferritin) are the main carriers of Al3+ ions in plasma and Al3+ ions can enter into the brain and reach the placenta and fetus. Al3+ ions may persist for a very long time in various organs and tissues before excretion through urine. Aluminum salts are neurotoxic and may be associated with Parkinson's disease3 and senile dementia (commonly known as Alzheimer's disease4), microcytic anemia, dialysis dementia and osteomalacia, and have even been shown to increase the risk of lung and bladder cancer.5–8 Aluminum should therefore be regarded as a toxic metal and its concentration in the environment should be monitored.In 1989 the WHO listed aluminum as a source of food pollution and limited its concentration in drinking water to 200 mg L−1 (7.41 mM). The FAO/WHO Joint Expert Committee on Food Additives recommended a maximum daily intake of aluminum of 3–10 mg per day per kg body mass. It is believed that almost 40% of the world's acid soils are affected by aluminum toxicity, which is a key factor affecting plant (i.e. crop) performance on acid soils.9,10 The detection of Al3+ ions is therefore crucial in controlling its concentration levels in environmental monitoring and its impact on human health.
Several conventional methods with moderate sensitivity for Al3+ ions have been developed with detection based on atomic absorption spectrometry (AAS) and chromatographic and spectrophotometric techniques.11 Of these methods, AAS requires expensive instrumentation and complicated sample preparation processes. Although other techniques have been developed for detecting trace amounts of Al3+ ions, most of these involve the use of harmful chemicals and the analytical procedures are easily interfered with by variations in pH and the coexistence of interfering ions. However, spectrofluorimetric methods have received considerable attention in recent years due to their simplicity, high sensitivity and real-time monitoring with a low response time.12–14
There are several fluorescent sensors for Al3+ ions with good selectivity, but this approach has several disadvantages, including complicated synthetic procedures, poor water solubility, insensitivity to biological systems, interference from other ions and variations with pH.15 The sensitive bioimaging of Al3+ ions in cells is a prerequisite for understanding the underlying mechanisms of how Al3+ ions cause aluminum-induced human diseases. Few reports have been published on chemosensors for the detection of Al3+ ions in aqueous media and most of these are based on chelation-enhanced fluorescence/photo-induced electron transfer.15 Very few published methods have used the fluorescence resonance energy transfer (FRET) mechanism.16 The sensors with fluorescence enhancement (a ‘turn-on’ response) through FRET are of considerable interest as FRET is a distance-dependent radiation-less transfer of energy from an excited donor fluorophore to a suitable acceptor fluorophore which can be used to investigate molecular level interactions.17 Therefore the development of new ratiometric FRET-based sensors for Al3+ ions with improved detection limits in the presence of water is desirable.
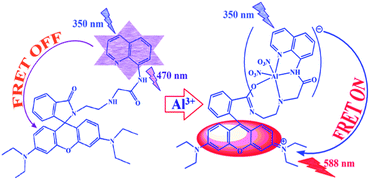
We report here a newly designed ratiometric FRET-based fluorescent sensor (L1) which is highly selective for Al3+ ions in HEPES buffer (1 mM, pH 7.4, 2% EtOH) at 25 °C. On excitation at 350 nm, the L1 probe exhibits a fluorescence maximum at 470 nm, which decreases along with a gradual increase in a new peak at 588 nm due to the addition of Al3+ ions. This phenomenon is a result of the ring opening of the spirolactam system of rhodamine, which gives rise to a strong fluorescence emission and a visual color change of the solution from colorless to pink. Ratiometric responses are attractive because the ratio between the two emission intensities can be used to measure the analyte concentration and the sensor molecule concentration, providing a built-in correction for environmental effects and giving stability under illumination.14,18 Interestingly, the presence of an excess of other metal ions, e.g. alkali (Na+, K+), alkaline earth (Mg2+, Ca2+), and transition metal ions (Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+) and Pb2+, does not affect the ‘switch-on’ behavior of the receptor L1 observed in the presence of Al3+ ions. Fluorescence microscopic studies confirmed that L1 could also be used as an imaging probe for the detection of the uptake of Al3+ ions in HeLa cells.
Experimental section
Materials and methods
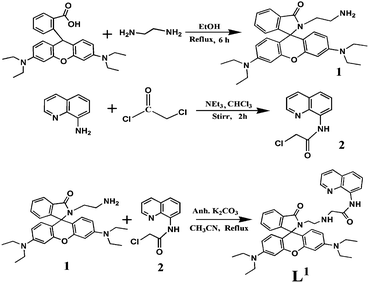
High-purity HEPES, 8-aminoquinoline, 2-chloroacetyl chloride and aluminum nitrate nonahydrate were purchased from Sigma Aldrich (India) and rhodamine B and ethylene diamine (en) from E. Merck. The solvents used were of spectroscopic grade. All metal salts were used as either their nitrate or chloride salts. Other chemicals were of analytical-reagent grade and used without further purification except where specified. Milli-Q, 18.2 MΩ cm−1 water was used throughout all experiments. A Shimadzu (Model UV-1800) spectrophotometer was used for recording electronic spectra. FTIR spectra were recorded using a Perkin Elmer FTIR Model RX1 spectrometer using a KBr disk. 1H-NMR spectra were obtained on a Bruker Avance DPX 500 MHz spectrometer and a Geol 400 MHz spectrometer using CDCl3 solution. Electrospray ionization (ESI) mass spectra were recorded on a Qtof Micro YA263 mass spectrometer. A Systronics digital pH meter (Model 335) was used to measure the pH of the solution and the pH was adjusted using either 50 mM HCl or NaOH solution. Steady-state fluorescence emission and excitation spectra were recorded with a Hitachi 4500 spectrofluorimeter. Time-resolved fluorescence lifetime measurements were obtained using a HORIBA JOBIN Yvon picosecond-pulsed diode laser based time-correlated single-photon counting spectrometer from IBH (UK) at λex = 377 nm with a micro-channel plate photomultiplier tube (MCP-PMT) as a detector. Emission from the sample was collected at right angles to the direction of the excitation beam maintaining magic angle polarization (54.71). The full width at half-maximum of the instrument response function was 250 ps and the resolution was 28.6 ps per channel. Data were fitted to multi-exponential functions after deconvolution of the instrument response function by an iterative reconvolution technique using IBH DAS 6.2 data analysis software in which reduced w2 and weighted residuals served as parameters for goodness of fit.Synthesis of the L1 probe
The L1 probe was synthesised using a three-step reaction process (Scheme 1). Rhodamine B-en carboxamide (1) was first prepared using a previously published method.19 Ethylene diamine (en, 4 mL) was added to a solution of rhodamine B (1 g, 2.09 mmol) in ethanol (40 mL). The solution was refluxed for 6 h. The reaction mixture was then evaporated under reduced pressure to give orange oil, which was subsequently recrystallized from methanol–water to give rhodamine B-en carboxamide (1) as a light orange crystal (77%).2-Chloro-N-(quinol-8-yl)acetamide (2) was then prepared from the reaction of 2-chloroacetyl chloride and 8-aminoquinoline. 2-Chloroacetyl chloride (5.31 mL) was dissolved in chloroform (5 mL) and then added dropwise to a cooled, stirred solution of 8-aminoquinoline (2.88 g, 20 mmol) and Et3N (3.0 mL) in chloroform (10 mL) within 1 h. After stirring for 2 h at room temperature the mixture was removed under reduced pressure to obtain a white solid, which was then filtered out and extracted with dichloromethane to give compound 2 (ref. 20) with a yield of 82% (m.p. 133 ± 2 °C).
In the final step, rhodamine B-en carboxamide (1) was taken in dry acetonitrile with anhydrous K2CO3. 2-Chloro-N-(quinolin-8-yl)acetamide (2) in dry acetonitrile was then added dropwise with stirring. The resulting reaction mixture was refluxed for 6 h. The volume of the solution was reduced to obtain a solid and then extracted with dichloromethane and finally purified by silica gel column chromatography using dichloromethane as the eluent. The yield was 73% (m.p. 103 ± 2 °C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, 2970; νC
C, 2970; νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1681; νC
O, 1681; νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, 1618. 1H-NMR (400 MHz, CDCl3): 10.91 (s, 1-NH–CO), 8.43 (dd, 1H), 8.21 (dd, 1H), 8.12 (dd, 1H), 7.45 (m, 2H), 7.08 (dd, 2H), 7.07 (m, 1H), 6.61 (dd, 2H), 6.06–6.14 (m, 7H), 3.56 (s, 2H, –CH2–CO), 3.31 (q, 8H, 4CH2), 2.84 (t, 2H), 2,49 (t, 2H), 1.06 (t, 12H, 4CH3). 13C-NMR (CDCl3): 166.18, 165.02, 154.18, 153.72, 149.53, 149.26, 148.99, 136.39, 133.69, 132.96, 131.60, 130.50, 129.32, 128.74, 127.59, 124.1, 123.57, 122.03, 116.90, 109.01, 108.48, 103.98, 98.19, 67.11, 44.80, 41.67, 40.54, 40.13, 13.04. ESI-MS m/z 669.0028 [M + H+, 60%], calc.: 669.347 [M + H+].
N, 1618. 1H-NMR (400 MHz, CDCl3): 10.91 (s, 1-NH–CO), 8.43 (dd, 1H), 8.21 (dd, 1H), 8.12 (dd, 1H), 7.45 (m, 2H), 7.08 (dd, 2H), 7.07 (m, 1H), 6.61 (dd, 2H), 6.06–6.14 (m, 7H), 3.56 (s, 2H, –CH2–CO), 3.31 (q, 8H, 4CH2), 2.84 (t, 2H), 2,49 (t, 2H), 1.06 (t, 12H, 4CH3). 13C-NMR (CDCl3): 166.18, 165.02, 154.18, 153.72, 149.53, 149.26, 148.99, 136.39, 133.69, 132.96, 131.60, 130.50, 129.32, 128.74, 127.59, 124.1, 123.57, 122.03, 116.90, 109.01, 108.48, 103.98, 98.19, 67.11, 44.80, 41.67, 40.54, 40.13, 13.04. ESI-MS m/z 669.0028 [M + H+, 60%], calc.: 669.347 [M + H+].Synthesis of L–Al complex as [Al(L)(NO3)2]
A solution of aluminum nitrate was added dropwise to a 10 mL ethanolic solution of L1 (0.01 mmol) and stirred for 4 h. The solvent was removed using a rotary evaporator and a blood red solid was obtained (Scheme S1†).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, 2845; νC
C, 2845; νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1691; νC
O, 1691; νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, 1612. 1H-NMR (500 MHz, CDCl3): 11.18 (s, 1-NH–CO), 8.76 (dd, 1H), 8.52 (dd, 1H), 8.11 (dd, 1H), 7.93 (dd, 1H), 7.87 (dd, 1H), 7.46 (dd, 2H), 7.39 (m, 1H), 7.08 (dd, 2H), 6.36 (t, 1H), 6.22 (d, 1H), 6.19 (d, 1H), 6.15 (d, 1H), 3.89 (s, 2H, –CH2–CO), 3.33 (q, 8H, 4CH2), 2.94 (t, 2H), 2.59 (t, 2H), 1.09 (t, 12H, 4CH3). 13C-NMR (DMSO-d6): 169.73, 169.10, 154.89, 154.50, 153.49, 150.12, 149.42, 134.44, 134.17, 130.23, 129.42, 129.11, 128.91, 127.88, 124.46, 124.15, 123.53, 123.23, 117.10, 109.15, 104.88, 104.68, 98.12, 47.02, 44.58, 38.64, 38.19, 13.20. ESI-MS in methanol: [M + CH3OH + H]+, m/z, 851.4242 (obs. with 11% abundance) (calc. m/z 851.85, where M = [Al(L)(NO3)2]).
N, 1612. 1H-NMR (500 MHz, CDCl3): 11.18 (s, 1-NH–CO), 8.76 (dd, 1H), 8.52 (dd, 1H), 8.11 (dd, 1H), 7.93 (dd, 1H), 7.87 (dd, 1H), 7.46 (dd, 2H), 7.39 (m, 1H), 7.08 (dd, 2H), 6.36 (t, 1H), 6.22 (d, 1H), 6.19 (d, 1H), 6.15 (d, 1H), 3.89 (s, 2H, –CH2–CO), 3.33 (q, 8H, 4CH2), 2.94 (t, 2H), 2.59 (t, 2H), 1.09 (t, 12H, 4CH3). 13C-NMR (DMSO-d6): 169.73, 169.10, 154.89, 154.50, 153.49, 150.12, 149.42, 134.44, 134.17, 130.23, 129.42, 129.11, 128.91, 127.88, 124.46, 124.15, 123.53, 123.23, 117.10, 109.15, 104.88, 104.68, 98.12, 47.02, 44.58, 38.64, 38.19, 13.20. ESI-MS in methanol: [M + CH3OH + H]+, m/z, 851.4242 (obs. with 11% abundance) (calc. m/z 851.85, where M = [Al(L)(NO3)2]).General method of UV-visible and fluorescence titration
The path length of the cells used for absorption and emission studies was 1 cm. For UV-visible and fluorescence titrations, a stock solution of L1 was prepared in HEPES buffer (1 mM, pH 7.4, 2% EtOH) at room temperature. Working solutions of L1 and Al3+ ions were prepared from their respective stock solutions. Fluorescence measurements were performed using a 5 nm × 5 nm slit width. All the fluorescence and absorbance spectra were taken after 30 minutes of mixing the Al3+ ions and L1 to acquire the optimized spectra.A series of solutions containing L1 and Al(NO3)3 was prepared such that the total concentration of L1 (10 μM) remained constant in all the sets. The mole fraction (X) of Al3+ ions was varied from 0.1 to 0.6. The absorbance at 561 nm was plotted against the mole fraction of the probe for stoichiometric determination.
Emission study
The organic moiety (L1) shows a very weak emission at 588 nm in HEPES buffer (1 mM, pH 7.4, 2% EtOH) at 25 °C when excited at 550 nm considering the absorption at 561 nm. Fluorescence quantum yields (Φ) were estimated by integrating the area under the fluorescence curves using the equation:where A is the area under the fluorescence spectral curve and OD is the optical density of the compound at the excitation wavelength of 550 nm and η is the refractive index of the solvent used. The standard used for the measurement of fluorescence quantum yield was rhodamine B (Φ = 0.7 in ethanol).
Calculation of Förster distance (R0)
The Förster distance (R0) for the FRET process was calculated from the following simplified equation:21,22where η is the refractive index (η = 1.33 in water),23 ΦD is the quantum yield of the donor, k denotes the average squared orientational part of a dipole–dipole interaction, typically k2 = 2/3,24 JDA is the degree of spectral overlap between the donor emission and the acceptor absorption, ID(λ) is the normalized fluorescence spectra of the donor and εA(λ) is the molar absorption coefficient of the acceptor.
Preparation of cell and in vitro cellular imaging with L1
Human cervical cancer cells (HeLa cell line) were purchased from the National Center for Cell Science, Pune, India and used throughout the study. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL) and a 1% antibiotic mixture containing penicillin, streptomycin and neomycin (PSN, Gibco BRL) at 37 °C in a humidified incubator with 5% CO2. For the experimental study, cells were grown to 80–90% confluence, harvested with 0.025% trypsin (Gibco BRL) and 0.52 mM EDTA (Gibco BRL) in phosphate-buffered saline (PBS, Sigma Diagnostics), plated at the desired cell concentration and then allowed to re-equilibrate for 24 h before treatment. Cells were rinsed with PBS and incubated with DMEM containing L1 (10 μM, 1% DMSO) for 30 min at 37 °C. All experiments were conducted in DMEM containing 10% FBS and 1% PSN antibiotic. The imaging system consisted of a fluorescence microscope (ZEISS Axioskop 2 plus) with an 10× objective lens.Cell cytotoxicity assay
To test the cytotoxicity of L1, an MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,S-diphenyl tetrazolium bromide] assay was performed using a previously published procedure.25 After treatment of the probe (5, 10, 20, 50 and 100 μM), 10 μL of MTT solution (10 mg mL−1 PBS) was added to each well of a 96-well culture plate and incubated continuously at 37 °C for 6 h. All media were removed from the wells and replaced with 100 μL of acidic isopropanol. The intracellular formazan crystals (blue-violet) formed were dissolved with 0.04 N acidic isopropanol and the absorbance of the solution was measured at 595 nm with a microplate reader. Data are given as the mean ± S.D. of three independent experiments. The cell cytotoxicity was calculated based on a cell viability of 100%.Results and discussion
Synthesis and characterization
Rhodamine B-en carboxamide (1) was obtained from rhodamine B using a previously published method19 and 2-chloro-N-(quinolin-8-yl)acetamide (2) was prepared from the reaction of 2-chloroacetyl chloride and 8-aminoquinoline in chloroform in the presence of NEt3 (Scheme 1). Probe L1 was then isolated from the reaction of rhodamine B-en carboxamide and 2-chloro-N-(quinolin-8-yl)acetamide in a dry acetonitrile solution in the presence of anhydrous K2CO3. The formulation of L1 was confirmed by physico-chemico and spectroscopic methods (Fig. S1A–D†). The L–Al complex was obtained when aluminum nitrate was mixed with an ethanolic solution of L1 with stirring. After removing the solvent, a blood red solid was obtained (Scheme S1†). The formulation of the L–Al complex was confirmed by physico-chemico and spectroscopic tools (Fig. S2A–D†).UV-visible spectroscopic studies of L1
The UV-visible spectra of L1 recorded in HEPES buffer (1 mM, pH 7.4, 2% EtOH) at 25 °C show an absorption maximum at 318 nm, which may be attributed to the intramolecular π–π* charge transfer transition. On the stepwise addition of Al3+ ions (0–30 μM) to the solution of L1 in HEPES buffer (1 mM, pH 7.4, 2% EtOH), the absorption intensity at 318 nm increased gradually and a new peak at 561 nm (Fig. 1) was generated due to the formation of a pink color from the colorless solution (Fig. 2). Considering the complexity of the intracellular environment, an additional experiment was performed with the probe to determine whether other ions were potential interferents. Metal ion selectivity assays were therefore performed while keeping the other experimental conditions unchanged. No significant change in the UV-visible spectral pattern was observed with the addition of 10 equivalents excess of the relevant metal ions, i.e. Na+, K+, Ca2+, Mg2+, Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Zn2+, Cd2+, Hg2+, Cu2+ and Pb2+. | ||
| Fig. 1 UV-visible titration spectra of L1 (10 μM) with the incremental addition of Al3+ ions (0–30 μM) in HEPES buffer (1 mM, pH 7.4, 2% EtOH). | ||
 | ||
| Fig. 2 Naked eye visual and fluorescence color changes in (A) probe L1 only and (B) in the presence of Al3+ ions in HEPES buffer (1 mM, pH 7.4, 2% EtOH). | ||
The complex formed between L1 and Al3+ was found to be of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, as established using the absorbance data with the help of Job's plot (Fig. S3†). Further confirmation of a 1
1 stoichiometry, as established using the absorbance data with the help of Job's plot (Fig. S3†). Further confirmation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with the probable formulation of an L–Al complex was established by the physico-chemical and spectroscopic data of the L–Al complex isolated in the solid form. The molecular ion peak in the ESI-MS spectra of L–Al3+ was observed at m/z 851.4242, evidence of the 1
1 stoichiometry with the probable formulation of an L–Al complex was established by the physico-chemical and spectroscopic data of the L–Al complex isolated in the solid form. The molecular ion peak in the ESI-MS spectra of L–Al3+ was observed at m/z 851.4242, evidence of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric species (Fig. S2B†).
1 stoichiometric species (Fig. S2B†).
Fluorescence spectroscopic studies of L1
To optimize the pH of the experimental conditions, a pH study was performed to control the efficiency of the probe (L1). In the absence of Al3+ ions, L1 showed a weak intensity fluorescence and an interesting independence of pH in the range 6.0–10.0 (Fig. S4†). At low pH the probe showed a high emission intensity because, at these pH values, the spirolactam ring opens irrespective of the species of metal ion added.18a,26 It was also noticed that the presence of Al3+ ions enhances the emission intensity of L1 significantly at pH 4.0–10.0. The emission spectrum of L1 excited at 350 nm shows a fluorescence maximum at 470 nm in HEPES buffer (1 mM, pH 7.4, 2% EtOH) at 25 °C. The intensities at 470 nm were significantly decreased with a concomitant increase in the intensities at 588 nm through an isoemissive point at ca. 553 nm when various concentrations of Al3+ ions (0–30 μM) were added (Fig. 3). | ||
| Fig. 3 Emission spectra of L1 (10 μM) in the presence of Al3+ ions (0–30 μM) at λex = 350 nm in HEPES buffer (1 mM, pH 7.4, 2% EtOH) at 25 °C. | ||
Ratiometric signaling of the fluorescence output at two different wavelengths plotted as a function of the concentration of Al3+ ions indicates that the fluorescence intensity ratio of wavelengths 470 and 588 nm (I588/I470) gradually increases with an increase in the concentration of Al3+ ions (Fig. 4). L1 showed an almost 75-fold increase in its fluorescence intensity with the addition of only 3.0 equivalents of Al3+ ions.
 | ||
| Fig. 4 Ratiometric signaling of fluorescence output at two different wavelengths plotted as a function of the concentration of Al3+ ions in aqueous HEPES buffer. | ||
From the plot of the fluorescence intensity at 588 nm (I588) versus [Al3+] it is suggested that L1 could be used to detect Al3+ ions as low as ca. 33 nM (Fig. S5†).27 To calculate the detection limit, a calibration graph (Fig. S6†) was obtained in the lower region. From the slope (S) of the graph and the standard deviation of seven replicate measurements at the zero level (σzero), the detection limit was estimated using the equation 3σ/S.17,28 This indicates that the detection limit of L1 for Al3+ ions is 6.19 × 10−9 M, which is comparable with the previously reported FRET-based Al3+ ion-selective fluorescent sensor,16b although our sensor is superior to the one reported previously because our FRET process takes place in aqueous solution.
A metal ion selectivity study was performed using L1 under identical experimental conditions to understand this phenomenon. Interestingly, the introduction of other metal ions causes the fluorescence intensity to be either unchanged or weakened. Fluorescence enhancement of L1 (10 μM) was not observed with the addition of an excess of 50 equivalents of biologically relevant metal ions, i.e. Na+, K+, Ca2+ and Mg2+ and 10 equivalents excess of several competitive metal ions (Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Zn2+, Cd2+, Hg2+, Cu2+ and Pb2+) (Fig. S7†); also no color change was seen with visual naked eye detection (Fig. S8 and S9†). Almost no adverse effect on intensity was observed in the presence of a 10 times excess of various tested ions with L1 and Al3+ ions (Fig. S10†).
As the receptor L1 has two different fluorophore units, we considered it appropriate to study the metal binding event of L1 at the two different excitation wavelengths corresponding to the xanthene unit (550 nm) and the quinoline unit (350 nm). Fig. 5 shows that the excitation of L1 at 550 nm in the absence of Al3+ did not initially show any significant emission in the range 550–700 nm, with a quantum yield of only 0.03. This supports the fact that the receptor remains in the spirolactam form in the absence of metal ions; the non-existence of the highly conjugated xanthene form results in the suppression of emission in this range of wavelengths. However, the addition of Al3+ ions to L1 induces a significant switch to an ON fluorescence response near 588 nm, showing a visual display of reddish fluorescence with a quantum yield of 0.61 (∼20-fold).
The switch to an ON response for the absorption spectral band at 561 nm and the emission band at 588 nm with binding to Al3+ ions suggests the opening of the spirolactam ring of L1 when metal ion coordination occurs (Scheme 2). It is observed that Al3+ ions bind to L1, inducing the ring opening of L1 and the generation of the xanthene moiety which is selective towards Al3+ ions. No noticeable spectral change was seen for the other metal ions tested.
The binding of Al3+ ions induces the opening of the spirolactam ring of L1 with an associated switch in the UV-visible spectral response in the range 380–650 nm, which has a significant spectral overlap with the emission spectrum of the N-(quinol-8-yl)-acetamide fragment (Fig. S11†). This suggests a plausible route for the non-radiative transfer of excitation energy from the donor quinoline to the acceptor xanthene moiety within the Förster critical distance (R0), calculated to be 20.03 Å, and initiates an intramolecular FRET process (viz. Scheme 2). In the free state of L1 the FRET pathway is totally suppressed and only an emission maximum near 470 nm was observed when L1 was excited at 350 nm. Binding of the receptor to Al3+ ions induces the FRET process to produce an intense rhodamine-based reddish emission, i.e. energy transfer from the N-(quinol-8-yl)-acetamide moiety to xanthene is due to the ring opening,29 resulting in an increase in the overlap integral between N-(quinol-8-yl)-acetamide and the xanthene moiety. Therefore, when titrated with Al3+ ions, the emission band at ca. 470 nm begins to decrease, along with a concomitant generation of a new fluorescence band at ca. 588 nm. This change in fluorescence was clearly visualised and the fluorescence color is significantly red (see Fig. 2).
The apparent binding constant (K) was determined using the modified Benesi–Hilderbrand method30 and was found to be 5.81 × 106 M−1 (Fig. S12†). The ring-opening phenomenon is also supported by the 13C-NMR analyses of L1 and the L–Al complex. In the 13C-NMR spectrum of L1 the signal at δ = 67.114 ppm, assignable to the tertiary carbon (sp3 hybridized) of the spirolactam ring in L1 (C6), is absent in the spectrum of the L–Al complex, but appears at δ = 134.44 ppm due to the conversion of C6 from sp3 to sp2 hybridized carbon; this feature supports the opening of the spirolactam ring (Fig. 6).31
In the 1H-NMR titration, a shift in the corresponding characteristic peaks into the downfield region was observed in support of the chelation of L1 in the solution state (Fig. S13†). The peaks at δ = 3.56 ppm assignable to –CH2–CO protons and at δ = 10.91 ppm assignable to NH–CO protons in the spectrum of L1 (Fig. S1C†) are significantly shifted to δ = 3.89 ppm and 11.18 ppm, respectively (Fig. S2C†).
The occurrence of the FRET process also agrees with the fluorescence lifetime data (Fig. 7 and Table 1). In the fluorescence lifetime experiments (λem = 470 nm), the average lifetime of L1 was 12.87 ns. After the addition of Al3+ ions to the solution of L1, the average lifetime (λem = 470 nm) of the complex species decreased to 11.15 and 8.37 ns, respectively, when the concentration of Al3+ ions was increased from 0.5 equivalent to 1.0 equivalent with respect to L1. In contrast, the average lifetime of the probe L1 increased from 1.60 to 9.77 ns (λem = 588 nm) when Al3+ ions were added to the solution of L1, which resembles the FRET process induced by Al3+ ions (Fig. S14†).
| λem = 470 nm | τav (ns) | χ2 | φ | kr (108 s−1) | knr (109 s−1) |
|---|---|---|---|---|---|
| L1 | 12.87 | 1.1 | 0.26 | 0.2020 | 0.0575 |
L1 + Al3+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
11.15 | 1.2 | — | — | — |
L1 + Al3+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
8.37 | 1.05 | 0.14 | 0.1673 | 0.1028 |
The values of kr and knr for the organic moiety L1 and the L–Al species are listed in Tables 1and 2 according to the equations32 τ−1 = kr + knr and kr = Φf/τ, where kr = the radiative rate constant and knr = total non-radiative rate constant. The data in Table 2 suggest that kr has only changed slightly; the factor that induces fluorescent enhancement is mainly ascribed to the decrease in knr.
| λem = 588 nm | τav (ns) | χ2 | φ | kr (108 s−1) | knr (109 s−1) |
|---|---|---|---|---|---|
| L1 | 1.6013 | 1.1 | 0.03 | 0.1873 | 0.60576 |
L1 + Al3+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
2.065 | 1.00 | — | — | — |
L1 + Al3+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
9.7729 | 1.05 | 0.61 | 0.6242 | 0.03990 |
Biological studies of L1 in the presence of Al3+
To examine the utility of the probe in biological systems, it was applied to the human cervical cancer HeLa cell line. In these experiments, both Al3+ ions and L1 were taken up by the cells and images of the cells were recorded by fluorescence microscopy following excitation at ∼550 nm. After incubation with L1 (10 μM) for 30 min, the cells displayed very faint intracellular fluorescence. However, the cells exhibited intensive fluorescence when exogenous Al3+ ions were introduced into the cell via incubation with an Al salt (Fig. 8). The fluorescence responses of the probe with various added concentrations of Al3+ ions are clearly evident from the cellular imaging. In addition, the in vitro study showed that 10 μM of L1 showed no cytotoxic effect on cells for up to 6 h (Fig. S15†). These results indicate that the probe has a huge potential as a sensor for Al3+ ions in both in vitro and in vivo applications.Conclusions
In summary, we conclude that this newly designed fluorescent chemosensor (L1) behaves as a highly specific and selective FRET-based ratiometric fluorescence probe towards Al3+ ions, which can also be detected by the naked eye. This fluorescence is due to the ring opening of the rhodamine spirolactam system. The detection limit of this L1 probe is very low (6.19 × 10−9 M) and it is comparable with the previously reported FRET-based Al3+ ion-selective fluorescent sensor. In this regard it may be considered as a superior FRET-based chemosensor for Al3+ ions in aqueous solution.16b This probe may be used as a biomarker for the imaging of Al3+ ions in living cells.Acknowledgements
Financial assistance from CSIR, New Delhi, India is gratefully acknowledged. B. Sen thanks UGC, New Delhi, India for offering him a fellowship. We sincerely acknowledge Professor Samita Basu and Mr Ajay Das, Chemical Science Division, SINP, Kolkata for enabling us to use the TCSPC instrument and we also thank Mr Samya Banerjee, IISC, Bangalore and Dr B. Mukherjee, IISER, Kolkata for recording the 1H-NMR, 13C-NMR and ESI–MS spectra.Notes and references
- M. G. Sont, S. M. White, W. G. Flamm and G. A. Burdock, Regul. Toxicol. Pharmacol., 2001, 33, 66 CrossRef PubMed.
- N. W. Bavlor, W. Egan and P. Richman, Vaccine, 2002, 20, S18–S23 CrossRef.
- D. P. Perl and A. R. Brody, Science, 1980, 208, 297 CAS.
- D. P. Perl, D. C. Gajdusek, R. M. Garruto, R. T. Yanagihara and C. J. Gibbs, Science, 1982, 217, 1053 CAS.
- E. H. Jeffery, K. Abreo, E. Burgess, J. Cannata, J. L. Greger and J. Toxicol, Environ. Health, 1996, 48, 649 CAS.
- A. Becaria, A. Campbell and S. C. Bondy, Toxicol. Ind. Health, 2002, 18, 309 CrossRef CAS.
- J. J. Spinelli, P. A. Demers, N. D. Le, M. D. Friesen, M. F. Lorenzi, R. Fang and R. P. Gallagher, Cancer Causes & Control, 2006, 17, 939 CrossRef PubMed.
- B. Armstrong, C. Tremblay, D. Baris and G. Thériault, Am. J. Epidemiol., 1994, 139, 250 CAS.
- E. Álvarez, M. L. Fernández-Marcos, C. Monterroso and M. J. Fernández-Sanjurjo, Application of aluminium toxicity indices to soils under various forest species, For. Ecol. Manage., 2005, 211, 227 CrossRef PubMed.
- J. Barceló and C. Poschenrieder, Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review, Environ. Exp. Bot., 2002, 48, 75 CrossRef.
- (a) H. Lian, Y. Kang, S. Bi, Y. Arkin, D. Shao, D. Li, Y. Chen, L. Dai, N. Gan and L. Tian, Talanta, 2004, 62, 43 CrossRef CAS; (b) H. Seol, S. C. Shin and Y. B. Shim, Electroanalysis, 2004, 16, 2051 CrossRef CAS; (c) O. Y. Nadzhafova, S. V. Lagodzinskaya and V. V. Sukhan, J. Anal. Chem., 2001, 56, 178 CrossRef CAS; (d) A. J. Downard, B. O'Sullivan and K. J. Powell, Anal. Chim. Acta, 1997, 345, 5 CrossRef CAS; (e) M. J. Ahmed and J. Hossan, Talanta, 1995, 42, 1135 CrossRef CAS.
- J. R. Lakowicz, Topics in Fluorescence Spectroscopy, in Probe Design and Chemical Sensing, Kluwer Academic Publishers, New York, 2002, vol. 4 Search PubMed.
- (a) A. P. de Silva, D. B. Fox, J. M. Huxley and T. S. Moody, Coord. Chem. Rev., 2000, 205, 41 CrossRef CAS; (b) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS.
- (a) S. Sen, T. Mukherjee, B. Chattopadhyay, A. Moirangthem, A. Basu, J. Marek and P. Chattopadhyay, Analyst, 2012, 137, 3975 RSC; (b) S. Sen, T. Mukherjee, S. Sarkar, S. K. Mukhopadhyay and P. Chattopadhyay, Analyst, 2011, 136, 4839 RSC; (c) U. C. Saha, K. Dhara, B. Chattopadhyay, S. K. Mandal, S. Mondal, S. Sen, M. Mukherjee, S. V. Smaalen and P. Chattopadhyay, Org. Lett., 2011, 13, 4510 CrossRef CAS PubMed; (d) U. C. Saha, B. Chattopadhyay, K. Dhara, S. K. Mandal, S. Sarkar, A. R. Khuda-Bukhsh, M. Mukherjee, M. Helliwell and P. Chattopadhyay, Inorg. Chem., 2011, 50, 1213 CrossRef CAS PubMed; (e) K. Dhara, U. C. Saha, A. Dan, M. Manassero, S. Sarkar and P. Chattopadhyay, Chem. Commun., 2010, 46, 1754 RSC; (f) M. Mukherjee, B. Sen, S. Pal, M. S. Hundal, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, RSC Adv., 2013, 3, 19978 RSC.
- (a) Neeraj, A. Kumar, V. Kumar, R. Prajapati, S. K. Asthana, K. K. Upadhyay and J. Zhao, Dalton Trans., 2014, 43, 583 RSC and references therein; (b) S. B. Maity and P. K. Bharadwaj, Inorg. Chem., 2013, 52, 1161 CrossRef CAS PubMed; (c) B. K. Datta, C. Kar, A. Basu and G. Das, Tetrahedron Lett., 2013, 54, 771 CrossRef CAS PubMed; (d) S. Goswami, A. Manna, S. Paul, K. Aich, A. K. Das and S. Chakraborty, Dalton Trans., 2013, 42, 8078 RSC; (e) D. Maity and T. Govindaraju, Chem. Commun., 2012, 48, 1039 RSC; (f) S. Kim, J. Y. Noh, K. Y. Kim, J. H. Kim, H. K. Kang, S.-W. Nam, S. H. Kim, S. Park, C. Kim and J. Kim, Inorg. Chem., 2012, 51, 3597 CrossRef CAS PubMed; (g) X. Sun, Y.-W. Wang and Y. Peng, Org. Lett., 2012, 14, 3420 CrossRef CAS PubMed; (h) Y. Lu, S. Huang, Y. Liu, S. He, L. Zhao and X. Zeng, Org. Lett., 2011, 13, 5274 CrossRef CAS PubMed; (i) M. Arduini, F. Felluga, F. Mancin, P. Rossi, P. Tecilla, U. Tonellato and N. Valentinuzzi, Chem. Commun., 2003, 1606 RSC.
- (a) A. Sahana, A. Banerjee, S. Lohar, A. Banik, S. K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Dalton Trans., 2013, 42, 13311 RSC; (b) A. Sahana, A. Banerjee, S. Lohar, B. Sarkar, S. K. Mukhopadhyay and D. Das, Inorg. Chem., 2012, 51, 11220 CrossRef PubMed; (c) M. Arduini, F. Felluga, F. Mancin, P. Rossi, P. Tecilla, U. Tonellato and N. Valentinuzzi, Chem. Commun., 2003, 1606 RSC.
- S. Pal, B. Sen, M. Mukherjee, K. Dhara, E. Zangrando, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, Analyst, 2014, 139, 1628 RSC.
- (a) B. Sen, M. Mukherjee, S. Pal, K. Dhara, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, RSC Adv., 2014, 4, 14919 RSC; (b) S. Sen, S. Sarkar, B. Chattopadhyay, A. Moirangthem, A. Basu, K. Dhara and P. Chattopadhyay, Analyst, 2012, 137, 3335 RSC; (c) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed; (d) Z. Xu, Y. Xiao, X. Qian, J. Cui and D. Cui, Org. Lett., 2005, 7, 889 CrossRef CAS PubMed.
- J. S. Wu, I.-C. Hwang, K. S. Kim and J. S. Kim, Org. Lett., 2007, 9, 907 CrossRef CAS PubMed.
- X. Zhou, P. Li, Z. Shi, X. Tang, C. Chen and W. Liu, Inorg. Chem., 2012, 51, 9226 CrossRef CAS PubMed.
- X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 2006, 443 Search PubMed.
- G. M. Hale and M. R. Querry, Appl. Opt., 1973, 12, 555 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 2006, p. 443 Search PubMed.
- J. Ratha, K. A. Majumdar, S. K. Mandal, R. Bera, C. Sarkar, B. Saha, C. Mandal, K. D. Saha and R. Bhadra, Mol. Cell. Biochem., 2006, 290, 113 CrossRef CAS PubMed.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed.
- A. Caballero, R. Martinez, V. Lloveras, I. Ratera, J. V. Gancedo, K. Wurst, A. Tarraga, P. Molina and J. Veciana, J. Am. Chem. Soc., 2005, 127, 15666 CrossRef CAS PubMed.
- A. Hakonen, Anal. Chem., 2009, 81, 4555 CrossRef CAS PubMed.
- (a) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed; (b) M. Vendrell, D. Zhai, J. C. Er and Y. T. Chang, Chem. Rev., 2012, 112, 4391 CrossRef CAS PubMed; (c) C. Kar, M. D. Adhikari, A. Ramesh and G. Das, Inorg. Chem., 2013, 52, 743 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- T. Mistri, R. Alam, M. Dolai, S. K. Mandal, P. Guha, A. R. Khuda-Bukhsh and M. Ali, Eur. J. Inorg. Chem., 2013, 2013, 5854 CrossRef CAS.
- N. J. Turro, Modern Molecular Photochemistry, Benjamin/Cummings Publishing Co., Inc, Menlo Park, CA, 1978 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Schemes, characterization data, tables, figures, and some spectra. See DOI: 10.1039/c4ra02378a |
| This journal is © The Royal Society of Chemistry 2014 |