Bio-imaging with neutral luminescent Pt(ii) complexes showing metal⋯metal interactions†
Abstract
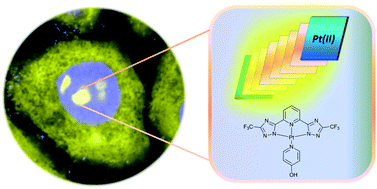
Molecular bio-imaging based on optical detection is facing important challenges in the attempt to develop new materials and small molecules able to have better emission quantum yield, stability toward photobleaching and long excited-state lifetime. A strategy to achieve these properties is to use triplet emitters based on metal complexes and to protect them from dioxygen quenching. We report on an interesting approach based on the use of self-assembled platinum compounds in order to obtain stable, highly emissive and long-lived species. Cell internalization and localization experiments show that the assemblies possess a different selectivity towards cellular compartments dictated by the terdentate ligand coordinated to the platinum. Also, the conditions used for the incubation determine cell internalization of the platinum complexes or their expulsion in the media.
- This article is part of the themed collections: Luminescence and photophysical properties of metal complexes and Cellular and Tissue Imaging – Luminescent Tags and Probes

 Please wait while we load your content...
Please wait while we load your content...