Easy access for the synthesis of 2-aryl 2,3-dihydroquinazolin-4(1H)-ones using gem-dibromomethylarenes as synthetic aldehyde equivalent†
Abstract
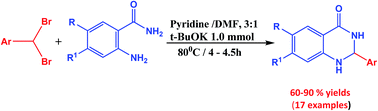
One step synthesis of 2,3-dihydroquinazolin-4(1H)-ones from gem-dibromomethylarenes using 2-aminobenzamide is described. Gem-dibromomethylarenes are used as aldehyde equivalent for the efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones, this synthesis takes shorter reaction time with quick isolation and excellent product yield.

 Please wait while we load your content...
Please wait while we load your content...